Prescribing Inflammatory Bowel Disease Medications in Chronic Kidney Disease: A Practical Guide
- PMID: 40601470
- PMCID: PMC12287904
- DOI: 10.1111/apt.70262
Prescribing Inflammatory Bowel Disease Medications in Chronic Kidney Disease: A Practical Guide
Abstract
Background: The prevalence of chronic kidney disease (CKD) in patients with inflammatory bowel disease (IBD) is increasing. The pharmacokinetic profiles of IBD medications in patients with advanced-stage CKD are not well studied.
Aim: To provide evidence-based guidance on the use of medical therapies in patients with IBD and CKD.
Methods: We conducted a narrative review of literature up to 31 March 2025 on studies of therapies currently used for the treatment of IBD in the setting of CKD, with a focus on advanced kidney disease and use in renal replacement therapy.
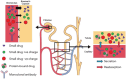
Results: Mesalazine can cause acute interstitial nephritis. Calcineurin inhibitors have been associated with nephrotoxicity. Methotrexate is contraindicated in advanced renal disease, including while on renal replacement therapy, due to higher risks of toxicity and myelosuppression. Dose adjustment of thiopurines should be considered in advanced renal disease due to metabolite accumulation. Monoclonal antibodies, including anti-tumour necrosis factor therapy, anti-integrin therapy and anti-interleukin 12/23 therapies, appear to be safe in renal insufficiency, including haemodialysis. There is limited data available for small molecule therapies; drug metabolism profiles suggest they are safe in CKD, although, for Janus kinase (JAK) inhibitors, including tofacitinib and upadacitinib, dose reduction should be considered in advanced renal disease.
Conclusion: Most therapies used in IBD, particularly biologic therapies, appear safe and effective when used in patients with CKD, including those on renal replacement therapy. Caution should be considered when using conventional therapies and JAK inhibitors.
Keywords: Crohn's disease; chronic kidney disease; dialysis; ulcerative colitis.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
References
-
- Stevens P. E., Ahmed S. B., Carrero J. J., et al., “KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease,” Kidney International 105, no. 4s (2024): S117–S314. - PubMed
-
- Ambruzs J. M. and Larsen C. P., “Renal Manifestations of Inflammatory Bowel Disease,” Rheumatic Disease Clinics of North America 44, no. 4 (2018): 699–714. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
