Financing drug development via adaptive platform trials
- PMID: 40601717
- PMCID: PMC12221166
- DOI: 10.1371/journal.pone.0325826
Financing drug development via adaptive platform trials
Abstract
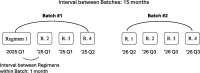
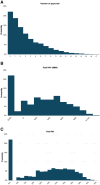
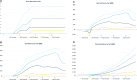
We propose a new approach to funding disease-specific drug development via a variation of the adaptive platform trial. This trial is designed to test a portfolio of drug candidates in parallel, with the cost of the trial partially covered by investors who receive payments from a royalty fund of the candidates in exchange for investment. Under realistic assumptions for cost, revenue, probability of success, drug sales, and royalty rates, investors may expect a return of 28%, but with a 22% probability of total loss. Such return distributions may be attractive to hedge funds, family offices, and philanthropic investors seeking both social impact and financial return. Return distributions palatable to mainstream investors may be achieved by funding multiple platform trials simultaneously and securitizing the aggregate cash flows.
Copyright: © 2025 Cho et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Andrew Lo reports personal investments in private biotech companies, biotech venture capital and mutual funds. He is a co-founder and partner of QLS Advisors, a healthcare analytics and investment company; a co-founder of BridgeBio Pharma, Gondola Bio, Quantile Health, Uncommon Cures; an advisor to Apricity Health, Aracari Bio, BrightEdge Impact Fund, Quantile Health, Think Therapeutics; and director of AbCellera, Atomwise, BridgeBio Pharma, n-Lorem, Uncommon Cures, Vesalius Therapeutics. Shomesh Chaudhuri and John Frishkopf are affiliated with QLS Advisors, a healthcare analytics and investment company. No other authors have competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Financial arrangements for health systems in low-income countries: an overview of systematic reviews.Cochrane Database Syst Rev. 2017 Sep 11;9(9):CD011084. doi: 10.1002/14651858.CD011084.pub2. Cochrane Database Syst Rev. 2017. PMID: 28891235 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

