Orbitofrontal cortex influences dopamine dynamics associated with alloparental behavioral acquisition in female mice
- PMID: 40601734
- PMCID: PMC12219497
- DOI: 10.1126/sciadv.adr4620
Orbitofrontal cortex influences dopamine dynamics associated with alloparental behavioral acquisition in female mice
Abstract
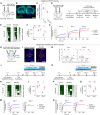
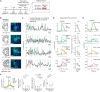
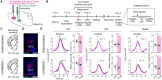
Maternal behaviors, which are crucial for the survival of mammalian infants, require the coordinated operation of multiple brain regions to process infant cues, make decisions, and execute motor plans. Although these processes likely demand higher cognitive functions, the prefrontal areas that regulate limbic parental programs remains poorly understood. Here, we show that the orbitofrontal cortex (OFC) excitatory projection neurons promote alloparental caregiving behaviors in female mice. By chronic microendoscopy, we observed robust yet adaptable representations of pup-directed anticipatory and motor-related activities within the OFC. Some of these plastic responses were significantly overlapped with those related to nonsocial reward signals. The inactivation of OFC output reduced the phasic activities of midbrain dopamine (DA) neurons specifically tied to pup retrieval and impaired the modulation of DA release to the ventral striatum during the acquisition of alloparental behaviors. These findings suggest that the OFC transiently boosts DA activity during the acquisition phase, thereby facilitating the manifestation of alloparental behaviors.
Figures




Similar articles
-
Neuromodulation of Dopamine D2 Receptors Alters Orbitofrontal Neuronal Activity and Reduces Risk-Prone Behavior in Male Rats with Inflammatory Pain.Mol Neurobiol. 2025 Jul;62(7):8187-8203. doi: 10.1007/s12035-025-04781-0. Epub 2025 Feb 22. Mol Neurobiol. 2025. PMID: 39985709 Free PMC article.
-
Multivariate Neural Representations of Value during Reward Anticipation and Consummation in the Human Orbitofrontal Cortex.Sci Rep. 2016 Jul 5;6:29079. doi: 10.1038/srep29079. Sci Rep. 2016. PMID: 27378417 Free PMC article.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
References
-
- Cohen L., Rothschild G., Mizrahi A., Multisensory integration of natural odors and sounds in the auditory cortex. Neuron 72, 357–369 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

