Nanosilicates promote angiogenesis through activation of ROS-mediated WNT/β-catenin pathway
- PMID: 40601740
- PMCID: PMC12219486
- DOI: 10.1126/sciadv.ado1223
Nanosilicates promote angiogenesis through activation of ROS-mediated WNT/β-catenin pathway
Abstract
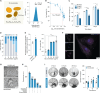
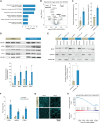
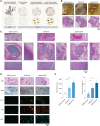
Scaffold vascularization plays a pivotal role in the wound healing process, facilitating the recruitment of endogenous progenitor cells and delivering crucial signaling molecules that promote cellular invasion, angiogenesis, and neotissue formation. In this study, we introduce nanosilicates as proangiogenic biomaterials that can prime endogenous cells to expedite angiogenesis and graft vascularization in vivo. Characterized by their mineral-based two-dimensional structure and extensive surface area, nanosilicates are efficiently internalized by endothelial cells, leading to augmented cellular migration and the formation of tubular structures. Through whole transcriptome sequencing, we elucidated that nanosilicates activate the canonical Wnt pathway through hypoxia-induced ROS production. In vivo investigations further corroborate the proangiogenic efficacy of nanosilicate-loaded biomaterials. This study posits nanosilicates as a potential proangiogenic adjunct in the design of biomaterials for in situ tissue regeneration.
Figures


References
-
- Xing Y., Yerneni S. S., Wang W., Taylor R. E., Campbell P. G., Ren X., Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials 281, 121357 (2022). - PubMed
-
- Briquez P. S., Clegg L. E., Martino M. M., Gabhann F. M., Hubbell J. A., Design principles for therapeutic angiogenic materials. Nat. Rev. Mater. 1, 15006 (2016).
-
- Gaharwar A. K., Singh I., Khademhosseini A., Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 5, 686–705 (2020).
-
- Arlov Ø., Rütsche D., Asadi Korayem M., Öztürk E., Zenobi-Wong M., Engineered sulfated polysaccharides for biomedical applications. Adv. Funct. Mater. 31, 2010732 (2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

