Growth arrest specific-6 and angiotoxin receptor-like signaling drive oral regenerative wound repair
- PMID: 40601775
- PMCID: PMC12310197
- DOI: 10.1126/scitranslmed.adk2101
Growth arrest specific-6 and angiotoxin receptor-like signaling drive oral regenerative wound repair
Abstract
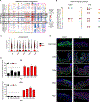
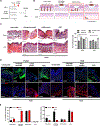
Rapid and scarless wound repair is a hallmark of the oral mucosa, yet the cellular and molecular mechanisms that enable this regeneration remain unclear. By comparing populations of murine oral mucosal fibroblasts (OMFs) and facial skin fibroblasts (FSFs), we have identified mechanisms that facilitate regeneration over fibrosis. We found that OMFs used growth arrest specific-6 (GAS6)-angiotoxin receptor-like (AXL) signaling to suppress fibrosis-related mechanosignaling through focal adhesion kinase (FAK) in vitro. Inhibition or knockdown of AXL in the murine oral mucosa resulted in fibrotic wounds and increased activation of FAK. Stimulation of AXL by exogenous GAS6 in the murine facial skin yielded wounds that healed regeneratively as assessed by collagen deposition and organization. Rare human oral scars that resulted from repetitive injury showed decreased expression of GAS6 and AXL and increased FAK. Activating AXL by exogenous GAS6 in repetitively injured mouse oral tissue resulted in better wound healing outcomes and reduced scarring. Altogether, we show that AXL signaling is necessary for murine regenerative wound healing in the oral mucosa and sufficient to limit facial skin fibrosis.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

