Design of a Mobile App and a Clinical Trial Management System for Cognitive Health and Dementia Risk Reduction: User-Centered Design Approach
- PMID: 40601924
- PMCID: PMC12268216
- DOI: 10.2196/66660
Design of a Mobile App and a Clinical Trial Management System for Cognitive Health and Dementia Risk Reduction: User-Centered Design Approach
Abstract
Background: The rising prevalence of dementia is a major concern, with approximately 45% of cases linked to 14 modifiable risk factors. The European project LETHE aims to develop a personalized digital intervention model to delay or prevent cognitive decline through risk factor management.
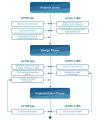
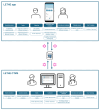
Objective: The objective of our study was to design a clinical trial platform for older individuals at risk of cognitive decline, including a mobile app for study participants and a clinical trial management system (CTMS) for health professionals.
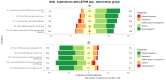
Methods: Using a user-centered design approach, workshops and feedback rounds involved potential participants representing the target group and professionals. The LETHE app's usability was assessed among 156 older adults enrolled in a 2-year multinational randomized controlled trial evaluating the feasibility of a digitally supported lifestyle program for dementia risk reduction. The randomized controlled trial is currently ongoing; the System Usability Scale (SUS) was administered 1 month after baseline to map first user experiences. Feedback on the LETHE CTMS was collected from 21 users.
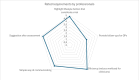
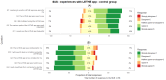
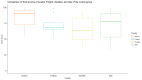
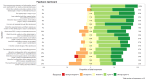
Results: Of the 78 participants in the trial intervention group, 66 (85%) provided responses for the mobile app, with a median SUS score of 70 (IQR 55-82). Within the control group, 73% (57/78) of responses were received, with a median SUS score of 73 (IQR 63-90). For the CTMS, we received 71% (15/21) of responses, and the feedback was mostly positive. A ranking of the features that could be considered beyond state of the art showed that the integration of personalized activities (mean 2.23, SD 1.17) and real-time appointments (mean 2.46, SD 1.51) were considered the most novel ones.
Conclusions: The LETHE app and CTMS were developed to support a personalized digital intervention method within a study involving 156 participants. Limitations include participants having digital literacy and internet access, potentially impacting the generalizability of the findings. Despite these limitations, positive feedback and high usability scores suggest promising potential for the LETHE app and CTMS in supporting personalized interventions to prevent cognitive decline in older adults.
Keywords: AI; artificial intelligence; clinical trial; cognitive decline; dementia; eHealth; health information systems; mHealth; medical informatics; mobile apps; mobile health; multidomain interventions; prevention.
©Hannes Hilberger, Bianca Buchgraber-Schnalzer, Simone Huber, Theresa Weitlaner, Markus Bödenler, Alara Abaci, Jeroen Bruinsma, Ana Diaz, Anna Giulia Guazzarini, Jenni Lehtisalo, Seungjune Lee, Vasileios Loukas, Francesca Mangialasche, Patrizia Mecocci, Tiia Ngandu, Anna Rosenberg, Elisabeth Stögmann, Konsta Valkonen, Elena Uhlik, Helena Untersteiner, Laura Kneß, Helmut Ahammer, Sten Hanke. Originally published in JMIR Aging (https://aging.jmir.org), 02.07.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


Similar articles
-
Exploring engagement patterns within a mobile health intervention for women at risk of gestational diabetes.Womens Health (Lond). 2025 Jan-Dec;21:17455057251327510. doi: 10.1177/17455057251327510. Epub 2025 Jun 5. Womens Health (Lond). 2025. PMID: 40470610 Free PMC article. Clinical Trial.
-
Designing Survey-Based Mobile Interfaces for Rural Patients With Cancer Using Apple's ResearchKit and CareKit: Usability Study.JMIR Form Res. 2024 Sep 26;8:e57801. doi: 10.2196/57801. JMIR Form Res. 2024. PMID: 39326043 Free PMC article.
-
Multi-domain interventions for the prevention of dementia and cognitive decline.Cochrane Database Syst Rev. 2021 Nov 8;11(11):CD013572. doi: 10.1002/14651858.CD013572.pub2. Cochrane Database Syst Rev. 2021. PMID: 34748207 Free PMC article.
-
Evaluating Effectiveness of mHealth Apps for Older Adults With Diabetes: Meta-Analysis of Randomized Controlled Trials.J Med Internet Res. 2025 Jun 17;27:e65855. doi: 10.2196/65855. J Med Internet Res. 2025. PMID: 40527504 Free PMC article.
-
Mobile health (m-health) smartphone interventions for adolescents and adults with overweight or obesity.Cochrane Database Syst Rev. 2024 Feb 20;2(2):CD013591. doi: 10.1002/14651858.CD013591.pub2. Cochrane Database Syst Rev. 2024. PMID: 38375882 Free PMC article.
References
-
- GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022 Feb;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. https://linkinghub.elsevier.com/retrieve/pii/S2468-2667(21)00249-8 S2468-2667(21)00249-8 - DOI - PMC - PubMed
-
- Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, Ames D, Banerjee S, Burns A, Brayne C, Fox NC, Ferri CP, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Nakasujja N, Rockwood K, Samus Q, Shirai K, Singh-Manoux A, Schneider LS, Walsh S, Yao Y, Sommerlad A, Mukadam N. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024 Aug 10;404(10452):572–628. doi: 10.1016/S0140-6736(24)01296-0.S0140-6736(24)01296-0 - DOI - PubMed
-
- Noach S, Witteman B, Boss HM, Janse A. Effects of multidomain lifestyle interventions on cognitive decline and Alzheimer's disease prevention: a literature review and future recommendations. Cereb Circ Cogn Behav. 2023;4:100166. doi: 10.1016/j.cccb.2023.100166. https://linkinghub.elsevier.com/retrieve/pii/S2666-2450(23)00010-7 S2666-2450(23)00010-7 - DOI - PMC - PubMed
-
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015 Jun 06;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5.S0140-6736(15)60461-5 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

