Treatment of colorectal peritoneal metastases with oxaliplatin induces biomarkers predicting response to immune checkpoint blockade
- PMID: 40602034
- PMCID: PMC12269567
- DOI: 10.1016/j.tranon.2025.102464
Treatment of colorectal peritoneal metastases with oxaliplatin induces biomarkers predicting response to immune checkpoint blockade
Abstract
Background: Colorectal cancer (CRC) patients with inoperable peritoneal metastases (PM) have a dismal prognosis with limited treatment options. Local treatment of CRC-PM with oxaliplatin is commonly applied, but biomarkers steering patient selection, or informing potentially effective combination therapies are lacking. A novel potentially effective treatment strategy is Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in which CRC-PM are exposed to cyclic treatment with high concentrations of locally applied oxaliplatin. However, it is unclear whether and how CRC-PM respond to PIPAC.
Methods: Here, we generated a biobank from 20 patients receiving PIPAC with oxaliplatin for CRC-PM. The biobank contains biopsies from 3 PM per patient, repeatedly sampled prior to each treatment cycle, and ascites. Anti-tumor effects were analyzed by shallow single-cell karyotype sequencing (sc-karyoSeq). RNA-sequencing and proteomics were performed to assess changes in gene and protein expression. Immunohistochemistry was performed to assess treatment-induced changes in tissue histology. Ascites was used to assess immunoglobulin content and reactivity.
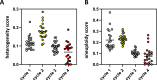
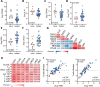
Results: PIPAC reduced genomic heterogeneity and aneuploidy scores among PIPAC-surviving tumor cells. Furthermore, PIPAC reduced immunosuppressive signals (hypoxia, interleukin-10, transforming growth factor β), and induced an influx of B and T lymphocytes, which organized into metastasis-associated Tertiary Lymphoid Structures (TLS). TLS are biomarkers predicting response to Immune-Checkpoint Inhibitors (ICIs). The T cells residing in PIPAC-induced TLS expressed high levels of the checkpoints PD-1, TIGIT and EBI3. PIPAC also caused the generation of plasma cells producing tumor-reactive antibodies.
Conclusion: PIPAC shows modest anti-tumor activity and induces immune parameters predicting response to ICIs. Patients with inoperable CRC-PM may therefore benefit from PIPAC in combination with ICIs.
Keywords: CRC; Colorectal; Heterogeneity; Karyotype; Oxaliplatin; PIPAC; Peritoneal metastasis; Reverse translation; Tertiary lymphoid structure.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Lurvink R.J., Rovers K.P., Nienhuijs S.W., Creemers G.J., Burger J.W.A., de Hingh I.H.J. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC-OX) in patients with colorectal peritoneal metastases-a systematic review. J. Gastrointest. Oncol. 2021;12(Suppl 1):S242–S258. - PMC - PubMed
-
- Alyami M., Hubner M., Grass F., Bakrin N., Villeneuve L., Laplace N., Passot G., Glehen O., Kepenekian V. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20(7):e368–e377. - PubMed
-
- Quenet F., Elias D., Roca L., Goere D., Ghouti L., Pocard M., Facy O., Arvieux C., Lorimier G., Pezet D., Marchal F., Loi V., Meeus P., Juzyna B., de Forges H., Paineau J., Glehen O., Group U.-G., Group B.I.G.R. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266. - PubMed
-
- van de Vlasakker V.C.J., Lurvink R.J., Cashin P.H., Ceelen W., Deraco M., Goere D., Gonzalez-Moreno S., Lehmann K., Li Y., Moran B., Morris D.L., Piso P., Quadros C.A., Rau B., Somashekhar S.P., Sommariva A., van der Speeten K., Spiliotis J., Sugarbaker P.H., Teo M.C.C., Verwaal V.J., Yonemura Y., Glehen O. de Hingh I. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: a web-based survey and 2021 statement by Peritoneal Surface Oncology Group International (PSOGI) J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021 - PubMed
LinkOut - more resources
Full Text Sources

