Anemoside B4 alleviates ulcerative colitis by attenuating intestinal oxidative stress and NLRP3 inflammasome via activating aryl hydrocarbon receptor through remodeling the gut microbiome and metabolites
- PMID: 40602277
- PMCID: PMC12271804
- DOI: 10.1016/j.redox.2025.103746
Anemoside B4 alleviates ulcerative colitis by attenuating intestinal oxidative stress and NLRP3 inflammasome via activating aryl hydrocarbon receptor through remodeling the gut microbiome and metabolites
Abstract
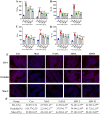
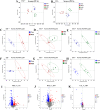
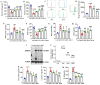
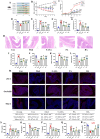
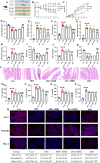
Ulcerative colitis (UC) is a chronic, non-specific inflammatory disease of the intestines with a significant increase in global incidence in recent years. Oxidative stress and inflammation are two hallmarks of UC pathogenesis. Anemoside B4 (AB4), a pentacyclic triterpenoid saponin, exhibits significant antioxidant and anti-inflammatory properties and shows potential for preventing UC. Here, an animal model induced by dextran sodium sulfate (DSS) was used to investigate the effect of AB4 on UC. The results demonstrated that AB4 significantly reduces intestinal oxidative stress and inflammation in UC mice, while also protecting intestinal barrier function. Furthermore, AB4 helps restore intestinal microbial balance primarily by modulating the abundance of Lactobacillus, which enhances the metabolism of short-chain fatty acids and upregulates the production of butyric acid (BA). Pseudogerm-free mice and fecal microbiota transplantation (FMT) demonstrated that AB4 significantly mitigated UC in a gut microbe-dependent manner. Both AB4 and BA markedly activate the aromatic hydrocarbon receptor (AhR). The intestinal organoid results suggest BA may activate the AhR to inhibit ROS production and activation of NLRP3 inflammasome, thereby protecting intestinal integrity. Administration of AhR antagonists abolished the protective effects, thus confirming the involvement of AhR in the underlying mechanism. Overall, these results indicate that AB4 is an effective agent against UC mainly by activating the AhR through gut microbial short-chain fatty acid metabolites to inhibit intestinal oxidative stress and inflammation.
Keywords: Anemoside B4; Aryl hydrocarbon receptor; Butyric acid; Gut microbiome; Oxidative stress; Ulcerative colitis.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012 Nov 3;380(9853):1606–1619. - PubMed
-
- Voelker R. What is ulcerative colitis? JAMA. 2024 Feb 27;331(8):716. - PubMed
-
- Eisenstein M. Ulcerative colitis: towards remission. Nature. 2018 Nov;563(7730):S33. - PubMed
-
- Wangchuk P., Yeshi K., Loukas A. Ulcerative colitis: clinical biomarkers, therapeutic targets, and emerging treatments. Trends Pharmacol. Sci. 2024 Oct;45(10):892–903. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

