Biomarker evidence of neurodegeneration in mid-life former rugby players
- PMID: 40602789
- PMCID: PMC12316018
- DOI: 10.1093/brain/awaf152
Biomarker evidence of neurodegeneration in mid-life former rugby players
Abstract
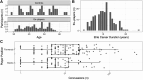
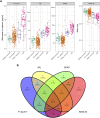
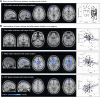
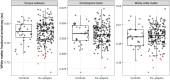
Repetitive head impacts and traumatic brain injuries in contact sports, such as rugby union, are associated with increased risk of neurodegenerative diseases such as Alzheimer's disease and chronic traumatic encephalopathy. Advances in fluid and imaging biomarkers are transforming dementia diagnosis but have not been systematically applied to individuals previously exposed to head impacts during rugby participation. We used biomarkers, including those with sensitivity and specificity for early Alzheimer's pathology to explore neurodegenerative risk in mid-life elite retired rugby players with significant repetitive head impact exposure. Plasma neurofilament light, glial fibrillary acid protein, amyloid-β (Aβ)42, Aβ40 and phospho-tau217 were quantified using ultrasensitive single molecule array digital enzyme-linked immunosorbent assay (SiMoA) in former elite rugby players as well as age/sex-matched unexposed controls. 3 T MRI and neuropsychology assessments were performed, with National Institute for Neurological Disorders and Stroke criteria used to ascertain the presence of traumatic encephalopathy syndrome. Regression models were used to relate plasma/imaging biomarkers to clinical phenotype. Individual-levels analyses were performed for fluid and imaging metrics, based on control biomarker distributions. Biomarker data from two aligned un-exposed Alzheimer's cohorts were included to contextualize our findings. Two hundred ex-rugby players (median age 44 years, 90% males) and 33 unexposed controls were assessed. Twenty-four (12%) ex-players fulfilled criteria for traumatic encephalopathy syndrome but none had dementia. Plasma phospho-tau217 concentrations were 17.6% higher in ex-rugby players than controls (95% confidence interval 3.7-33.3, P = 0.047). A total of 46 (23.1%) ex-players had elevated phospho-tau217 at the individual level; as did 18 (9.0%) players in relation to raised plasma neurofilament light. Ex-players' concentrations were lower than in unexposed adults with late onset Alzheimer's disease (n = 69). Ex-players also showed significantly reduced volumes in the frontal/cingulate cortex on voxel-based morphometry at the group level; with reduced white matter and lower hippocampal volume associated with longer career durations within ex-players. Trauma-associated white matter changes measured with diffusion tensor imaging were uncommon in ex-players (4.6%). Traumatic encephalopathy syndrome was significantly more common in ex-players with elevated phospho-tau217, while those with raised plasma neurofilament light had significantly more anxiety and depressive symptoms. Frontal brain volumes correlated negatively with neurofilament light (r = -0.21, P = 0.010), and hippocampal volumes correlated negatively with phospho-tau217 (r = -0.19, P = 0.024). Elite rugby participation is associated with abnormal fluid and neuroimaging neurodegeneration biomarkers in mid-life. These include elevated phospho-tau217, which may indicate amyloid-dependent tau pathology. The results provide support for using state-of-the-art neurodegenerative biomarkers in the evaluation of long-term effects of sports head impact exposure.
Keywords: CTE; TBI; concussion; dementia; head injury; sports.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
D.J.S. has received support of blood biomarker analysis (unrelated to this paper) by Quanterix and Alamar. D.J.S. and R.J.S. participate in an expert panel advising the English Rugby Football Union on concussion and related issues. D.J.S. has received an honorarium from the Rugby Football Union for his participation, which has been used for research purposes. R.J.S. acts as an independent concussion expert for World Rugby and receives payment for providing independent clinical care for professional players following head injuries. D.J.S. and R.S. have received complementary tickets to international rugby matches paid for by the Rugby Football Union. ISEH invoices the Rugby Football Union for the number of ex-players seen in clinic per quarter. In turn, D.J.S., R.S., D.F. and M.P. receive pass through payment from ISEH for the provision of private clinical services. ISEH receives funding from the RFU and FA to employ E.J.R. and Y.L. for the provision of research services. Imperial College London receive funding from the RFU and FA to support the employment of J.A.H., K.A.Z., M.D.G. for the provision of research services. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside the submitted work).
Figures
References
-
- Hammond FM, Hart T, Bushnik T, Corrigan JD, Sasser H. Change and predictors of change in communication, cognition, and social function between 1 and 5 years after traumatic brain injury. J Head Trauma Rehabil. 2004;19:314–328. - PubMed
MeSH terms
Substances
Grants and funding
- NIHR Academic Clinical Lectureships
- NIHR Imperial Biomedical Research Centre
- #2023-00356/Swedish Research Council
- #2022-01018/Swedish Research Council
- #2019-02397/Swedish Research Council
- 101053962/Swedish Research Council
- #ALFGBG-71320/Swedish Research Council
- #201809-2016862/Alzheimer's Drug Discovery Foundation
- #ADSF-21-831376-C/Alzheimer's Drug Discovery Foundation
- #ADSF-21-831381-C/Alzheimer's Drug Discovery Foundation
- #ADSF-21-831377-C/Alzheimer's Drug Discovery Foundation
- #ADSF-24-1284328-C/Alzheimer's Drug Discovery Foundation
- #22HLT07/European Partnership on Metrology
- Cure Alzheimer's Fund
- Olav Thon Stiftelsen
- Erling-Persson Family Foundation
- #FO2022-0270/Stiftelsen för Gamla Tjänarinnor
- 860197/H2020 Marie Skłodowska-Curie Actions
- JPND2021-00694/H2020 Marie Skłodowska-Curie Actions
- University College London Hospitals Biomedical Research Centre
- UKDRI-1003/UK Dementia Research Institute
- MR/W016095/1/MRC_/Medical Research Council/United Kingdom
- ALZS_/Alzheimer's Society/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

