Self-Assembly of Accumulated Sphingolipids into Cytotoxic Fibrils in Globoid Cell Leukodystrophy and Their Inhibition by Small Molecules In Vitro
- PMID: 40603002
- PMCID: PMC12269364
- DOI: 10.1021/acsnano.5c05498
Self-Assembly of Accumulated Sphingolipids into Cytotoxic Fibrils in Globoid Cell Leukodystrophy and Their Inhibition by Small Molecules In Vitro
Erratum in
-
Correction to "Self-Assembly of Accumulated Sphingolipids into Cytotoxic Fibrils in Globoid Cell Leukodystrophy and Their Inhibition by Small Molecules in Vitro".ACS Nano. 2025 Sep 23;19(37):33665. doi: 10.1021/acsnano.5c14040. Epub 2025 Sep 10. ACS Nano. 2025. PMID: 40930988 Free PMC article. No abstract available.
Abstract
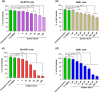
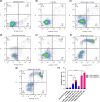
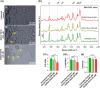
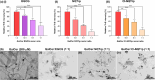
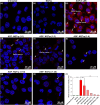
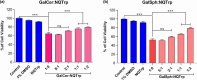
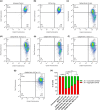
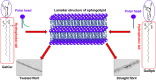
Globoid cell leukodystrophy (GLD) is a rare hereditary inborn error of metabolism due to recessive mutations that cause loss of function of the enzyme galactosylceramidase (GALC). This results in the accumulation of the sphingolipids galactosylceramide (GalCer) and galactosylsphingosine (GalSph) in the lysosomes of neuronal cells. The accumulated GalCer and GalSph in cerebral macrophages of GLD patients are neurotoxic to oligodendrocytes and Schwann cells, leading to demyelination in the nervous system. The disease typically presents with infantile onset in the first six months of life and death by age 2. Here, we identified a supramolecular structure of GalCer and GalSph that may contribute to GLD pathology. Using biophysical assays commonly used for studying proteinaceous amyloids, e.g., amyloid-specific dyes, microscopical imaging, and a series of analytical methods (FTIR, PXRD, and SAXS), we demonstrate that both GalCer and GalSph can self-assemble in vitro into highly organized fibrils reminiscent of fibrils of amyloidogenic proteins. These fibrils exhibit significant cytotoxicity to both neuronal and oligodendroglial cells. Using an inhibitor of the GALC enzyme in cell culture to mimic the GLD pathophysiology, we could detect the accumulation of these fibrils in cells. We also observed that small molecules, which are bona fide inhibitors of proteinaceous amyloids, effectively mitigated the formation of the GalCer and GalSph fibrillar structures in vitro. Finally, the small molecule ameliorated the cytotoxic effects of the sphingolipid fibrils in SH-SY5Y cells, suggesting a potential avenue for therapeutic intervention in GLD orphan disease.
Keywords: apoptosis; galactosylceramide; galactosylsphingosine; globoid cell leukodystrophy; self-assembly; small molecule inhibitors; sphingolipids.
Figures