AKR1C3 enhances radioresistance in esophageal adenocarcinoma via inhibiting ferroptosis through suppressing TRIM21-mediated ubiquitination of HSPA5
- PMID: 40603306
- PMCID: PMC12222831
- DOI: 10.1038/s41419-025-07773-z
AKR1C3 enhances radioresistance in esophageal adenocarcinoma via inhibiting ferroptosis through suppressing TRIM21-mediated ubiquitination of HSPA5
Abstract
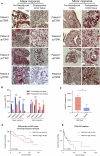
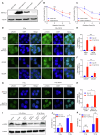
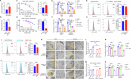
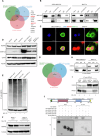
Esophageal adenocarcinoma (EAC) is the predominant subtype of esophageal cancer (EC) in high-income countries, and radioresistance is one of the key factors for the poor prognosis. In this study, we successfully established a radioresistant EAC in vitro model. Aldo-keto reductase 1C3 (AKR1C3) was identified as a promising regulator of radioresistance by RNA-seq analysis and subsequent functional studies. Through integrated analyses of scRNA-seq and TCGA datasets, we found that AKR1C3 was likely to enhance radioresistance by inhibition of ferroptosis. Indeed, analysis of the lipid ROS level by C11-Bodipy staining and the result of transmission electron microscopy revealed that AKR1C3 could prevent EAC cells from ferroptosis. Mechanistically, AKR1C3 binds to the nucleotide-binding domain of HSPA5, thereby inhibiting the E3 ligase TRIM21-induced ubiquitin-dependent proteasomal degradation of HSPA5, which further stabilizes GPX4, thus inhibiting ferroptosis. Importantly, AKR1C3 inhibitor resensitized the EAC patient-derived organoids to radiotherapy. In conclusion, this study highlights AKR1C3 as a regulator of radioresistance and a potential therapeutic target in EAC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the Institutional Ethics Committee of the University Hospital of Cologne (ID: 13-091 and ID:18-274).
Figures


References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Joseph A, Raja S, Kamath S, Jang S, Allende D, McNamara M, et al. Esophageal adenocarcinoma: a dire need for early detection and treatment. Cleve Clin J Med. 2022;89:269–79. - PubMed
-
- Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. - PubMed
-
- van Laarhoven H, Verhoeven R, van Berge Henegouwen M, Mohammad NH, van Hillegersberg R, Slingerland M, et al. Real-world outcomes of the CROSS regimen in patients with resectable esophageal or gastro-esophageal junction adenocarcinoma: a nationwide cohort study in the Netherlands. EClinicalMedicine. 2025;80:103067. - PMC - PubMed
-
- Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63:232–48. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

