Oleic acid restores the impaired antitumor immunity of γδ-T cells induced by palmitic acid
- PMID: 40603316
- PMCID: PMC12222472
- DOI: 10.1038/s41392-025-02295-8
Oleic acid restores the impaired antitumor immunity of γδ-T cells induced by palmitic acid
Abstract
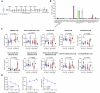
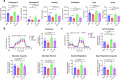
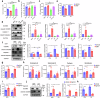
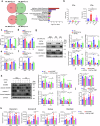
Dietary fatty acids (FAs) are associated with the therapeutic intervention under various health conditions. Human γδ-T cells are indispensable for immunosurveillance toward malignant cells. However, their impact on γδ-T cell metabolism and function remains poorly unexplored. Here, we applied targeted metabolomics analysis to serum FAs among cancer patients undergoing γδ-T cell therapy and discovered that palmitic acid (PA) or oleic acid (OA) levels were associated with the efficacy of Vγ9Vδ2-T cell therapy. We further elucidated that PA suppresses the antitumor activity of Vγ9Vδ2-T cells by disrupting metabolic processes and inhibiting the secretion of lytic granules, whereas OA restores the impaired antitumor activity of Vγ9Vδ2-T cells. Mechanistically, we surprisingly found that PA stimulates Vγ9Vδ2-T cells to secrete excessive IFNγ, which in turn induces cell pyroptosis, ultimately resulting in decreased antitumor activity. In contrast, OA reduces IFNγ secretion and mitigates cell pyroptosis, thereby restoring their antitumor activity. Alternatively, direct blockade of IFNγ by anti-IFNγ mAb or inhibition of pyroptosis by dimethyl fumarate (DMF) also restores their antitumor activity. This study highlights a novel mechanism whereby dietary FAs modulate γδ-T cell function through regulating IFNγ-mediated pyroptosis. Additionally, it offers proof-of-concept for an innovative approach by targeting IFNγ-mediated pyroptosis or dietary OA supplementation to strengthen the antitumor immunity of γδ-T cells against cancers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Nava Lauson, C. B. et al. Linoleic acid potentiates CD8(+) T cell metabolic fitness and antitumor immunity. Cell Metab.35, 633–650.e639 (2023). - PubMed
-
- Lai, Y. et al. Dietary elaidic acid boosts tumoral antigen presentation and cancer immunity via ACSL5. Cell Metab.36, 822–838.e828 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

