Detection of caffeic acid in honey using carbon paste electrode modified by copper (II) oxide (CuO) nanoparticles
- PMID: 40603340
- PMCID: PMC12222804
- DOI: 10.1038/s41598-025-04245-4
Detection of caffeic acid in honey using carbon paste electrode modified by copper (II) oxide (CuO) nanoparticles
Abstract
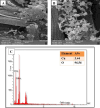
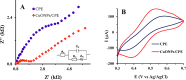
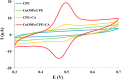
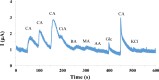
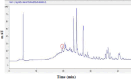
Identification of caffeic acid (CA) in food such as honey is of interest as it has antioxidant, antibacterial, anti-inflammatory, anti-fungal, etc. properties. Accordingly it is necessary to provide a simple, fast, sensitive and low-cost method for CA determination. Here, the carbon paste electrode surface was modified with CuO nanoparticles to obtain CuONPs/CPE as an electrochemical sensor for CA. The CuONPs were synthesized via sol-gel method and characterized using field emission scanning electron microscopy (FE-SEM), energy dispersive X-ray (EDX), X-ray diffraction (XRD) and fourier-transform infrared spectroscopy (FT-IR). Electrochemical behavior of CA at the CPE and CuONPs/CPE surfaces was studied using cyclic voltammetry (CV) technique in phosphate buffered saline (PBS; 0.1 M, pH 2.3). The oxidation and reduction peaks of CA were observed at 0.49 (EPa) and 0.47 (EPc) V (vs. Ag/AgCl, 3.0 M KCl). Differential pulse voltammetry (DPV) was employed to measure CA, which showed a linear response range from 5.0 to 50.0 µM, with a limit of detection (LOD) and a limit of quantification (LOQ) as 3.21 and 10.7 µM, respectively. In real samples of Thyme and Astragalus honeys, CA was measured at 4.96 and 2.89 mg per g of honey, respectively. In order to comparison, the CA in Astragalus honey was determined with HPLC technique. The fabricated electrochemical sensor (CuONPs/CPE), with the ability to directly detect CA in honey samples, simplicity, rapid response, and cost-effectiveness, can be a suitable candidate for CA identification in food and beverage products.
Keywords: Caffeic acid; Carbon paste; Copper (II) oxide nanoparticles; Honey; Voltammetry.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures










References
-
- Leyva-Jimenez, F. J., Lozano-Sanchez, J., Borras-Linares, I., Cadiz-Gurrea, M. L. & Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against gram positive and gram negative bacteria. LWT101, 236–245 (2019). - DOI
-
- León-Ruiz, V., Vera, S., González-Porto, A. V., San, M. P. & Andrés Analysis of water-soluble vitamins in honey by isocratic RP-HPLC. Food. Anal. Methods. 6 (2), 488–496 (2013). - DOI
-
- Amiot, M. J., Aubert, S., Gonnet, M. & Tacchini, M. Les composés phénoliques des miels: étude préliminaire Sur l’identification et La quantification par familles. Apidologie20 (2), 115–125 (1989). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

