New tactics in the design of theranostic radiotracers
- PMID: 40603655
- PMCID: PMC12118753
- DOI: 10.1038/s44303-024-00027-1
New tactics in the design of theranostic radiotracers
Abstract
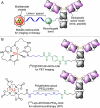
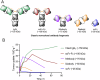
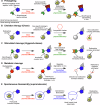
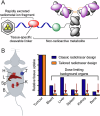
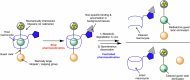
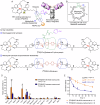
In the context of molecularly targeted radiotherapy, dosimetry concerns in off-target tissues are a major limitation to the more wide-spread application of radiopharmaceuticals to treat diseases like cancer. Reducing off-target accumulation of radionuclides in background tissues, whilst maintaining high and specific uptake in disease sites and improving the therapeutic window, requires rethinking common radiotracer design concepts. This article explores ways in which innovative radiotracer chemistry (the making and breaking of bonds) is used to modify interactions with the host organism to control excretion profiles and dosimetry at the tissue-specific level.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Research, P. Nuclear Medicine Market. https://www.precedenceresearch.com/nuclear-medicine-market/ ‘The global nuclear medicine market, forecast period 2024 to 2033.’ (2024).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
