Comparative effectiveness of teriflunomide and ocrelizumab on smoldering activity in multiple sclerosis: an observational study in the Swiss Multiple Sclerosis Cohort
- PMID: 40603656
- PMCID: PMC12222313
- DOI: 10.1007/s00415-025-13221-x
Comparative effectiveness of teriflunomide and ocrelizumab on smoldering activity in multiple sclerosis: an observational study in the Swiss Multiple Sclerosis Cohort
Abstract
Background: This study aimed to compare the effects of teriflunomide and ocrelizumab on clinical and MRI endpoints related to smoldering activity in relapsing-remitting multiple sclerosis (RRMS).
Methods: In this observational, longitudinal, multicenter study, we included 128 people with RRMS (pwRRMS) treated with teriflunomide and 495 treated with ocrelizumab. Outcomes included time to progression independent of relapse activity (PIRA). In a subset, we also assessed brain volume loss (BVL), longitudinal changes in diffusion tensor imaging (DTI) metrics, and the burden of paramagnetic rim lesions (PRLs). Propensity score matching was used for between-group comparisons.
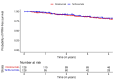
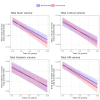
Results: Over a median follow-up of 3.1 years in the ocrelizumab group and 1.9 years in the teriflunomide group, there were no significant differences in the risk of PIRA (HR for teriflunomide vs. ocrelizumab: 0.80 [95%-CI:0.40-1.60]; p = 0.53). PwRRMS treated with teriflunomide exhibited lower annualized rates of BVL (-0.80 [95%-CI: -0.91; -0.69] vs. -1.06 [95%-CI: -1.25; -0.86]; p = 0.025) and gray matter volume loss (-0.92 [95%-CI: -1.05; -0.79] vs. -1.20 [95%-CI: -1.43; -0.97]; p = 0.035). No differences were observed in DTI metrics or PRL count.
Conclusions: This real-world study suggests that teriflunomide shows similar efficacy to ocrelizumab on smoldering activity, with a potentially greater effect in reducing BVL. Further research is needed to confirm these findings and understand their long-term implications.
Keywords: Disease-modifying therapies; Ocrelizumab; PIRA; PRLs; Smoldering MS; Teriflunomide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: A. Cagol has received speaker honoraria from Novartis and Roche. S. Schadelin, M. Ocampo-Pineda, P. Benkert, L. Melie-Garcia, L. Luchetti, C. Pot, S. Finkener, G. Disanto, J. M. Lieb, F. Wagner, O. Chan-Hi Kim have nothing to disclose. Ö. Yaldizli received grants from ECTRIMS/MAGNIMS, University of Basel, Pro Patient Stiftung University Hospital Basel, Free Academy Basel, Swiss Multiple Sclerosis Society and advisory board/lecture and consultancy fees from Roche, Sanofi Genzyme, Allmirall, Biogen and Novartis. J. Oechtering received research support by the Swiss MS Society and served on advisory boards for Roche and Merck. M. D’Souza, Michael Diepers, R. Du Pasquier, M. Battaglini have nothing to disclose in relation to this work. B. Fisher-Barnicol reported personal fees from Biogen outside the submitted work. S. Müller received honoraria for travel, honoraria for lectures/consulting, and/or grants for studies from Almirall, Biogen, Celgene, Novartis, Teva, Merck Serono, Genzyme, Roche, and Bayer Schweiz. J. Vehoff received honoraria for travel, honoraria for lectures/consulting and/or grants for studies from Allmiral, Biogen, Novartis, Teva, Merck-Serono, Genzyme, Roche and Bayer Schweiz AG, none related to this work. A. Chan has served on advisory boards for, and received funding for travel or speaker honoraria from, Actelion-Janssen, Almirall, Bayer, Biogen, Celgene, Sanofi-Genzyme, Merck, Novartis, Roche, and Teva, all for hospital research funds; and research support from Biogen, Genzyme and UCB. A. Chan is associate editor of the European Journal of Neurology and serves on the editorial board for Clinical and Translational Neuroscience and as topic editor for the Journal of International Medical Research. C. Zecca’ institution the Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland receives financial support from Teva, Merck Serono, Biogen, Genzyme, Roche, Celgene, Bayer and Novartis. T. Derfuss received speaker fees, research support, travel support, and/or served on Advisory Boards or Steering Committees of Alexion, Biogen, Celgene, GeNeuro, MedDay, Merck, Novartis, Roche and Sanofi-Genzyme; he received research support from Alexion, Biogen, Novartis, Roche, Swiss National Research Foundation, University of Basel, and Swiss MS Society. P. H. Lalive received honoraria for speaking and or travel expense from Biogen, Merck, Novartis, Roche; consulting fees from Biogen, GeNeuro, Merck, Novartis, Roche; research support from Biogen, Merck, Novartis. None were related to this work. E. Pravatà received honoraria for serving on scientific advisory boards from Bayer AG, unrelated to the present work. R. Hoepner received speaker/advisor honorary from Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Janssen, Bristol-Myers Squibb, Teva/Mepha and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi, Biogen, Chiesi, and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society, the SITEM Insel Support Fund and is a member of the Advisory Board of the Swiss and International MS Society. He also serves as deputy editor in chief for Journal of Central Nervous System disease and is part of the ECTRIMS Young Investigator Committee. P. Roth has received honoraria for lectures or advisory board participation from Alexion, Bristol-Myers Squibb, Boehringer Ingelheim, CDR-Life, Debiopharm, Galapagos, Laminar, Midatech Pharma, Novocure, OM Pharma, QED, Roche, Sanofi and Servier and research support from Merck Sharp and Dohme and TME Pharma. C. Gobbi’s institution, the Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland received financial support from Teva, Merck Serono, Biogen, Genzyme, Roche, Celgene, Bayer and Novartis. PHL received honoraria for speaking from Biogen-Idec, CSL Bering, Merck Serono, Novartis, Sanofi-Aventis, Teva; consulting fees from Biogen-Idec, Geneuro, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, Teva; research grants from Biogen-Idec, Merck Serono, Novartis. D. Leppert is Chief Medical Officer of GeNeuro. L. Kappos has received no personal compensation. His institutions (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support: payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from: Actelion, Bayer, BMS, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, Glaxo Smith Kline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hoffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera, and license fees for Neurostatus-UHB products; grants from Novartis, Innosuisse, and Roche. M. P. Sormani received consulting fees from Biogen, Merck, Novartis, Roche, Sanofi, Immunic, Alexion. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_212534/1), University of Basel, Progressive MS Alliance, Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi, Stata DX. C. Granziera: The University Hospital Basel (USB) and the Research Center for Clinical neuroimmunology and Neuroscience (RC2NB), as the employers of Cristina Granziera, have received the following fees which were used exclusively for research support from Siemens, GeNeuro, Genzyme-Sanofi, Biogen, Roche. They also have received advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro, Merck, Biogen and Roche; as well as speaker fees from Genzyme-Sanofi, Novartis, GeNeuro, Merck, biogen and Roche.
Figures

References
-
- Scalfari A, Traboulsee A, Oh J et al (2024) Smouldering-associated worsening in multiple sclerosis: an international consensus statement on definition, biology, clinical implications, and future directions. Ann Neurol 96(5):826–845. 10.1002/ANA.27034 - PubMed
-
- Müller J, Cagol A, Lorscheider J et al (2023) Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA Neurol 80(11):1232–1245. 10.1001/jamaneurol.2023.3331 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

