L-arginine vs. L-glutamine oral suspensions for radiation-induced oral mucositis: a triple-blind randomized trial
- PMID: 40603739
- PMCID: PMC12222376
- DOI: 10.1007/s00432-025-06213-x
L-arginine vs. L-glutamine oral suspensions for radiation-induced oral mucositis: a triple-blind randomized trial
Abstract
Purpose: Radiation-induced oral mucositis (RIOM) severely impacts patients with head and neck cancer (HNC) undergoing radiotherapy, often leading to pain and malnutrition. L-arginine and glutamine are immune-enhancing amino acids with potential benefits in wound healing and inflammation control. This study evaluated the efficacy of L-arginine versus L-glutamine oral suspensions in managing RIOM.
Methods: In this triple-blind, randomized controlled trial, 69 HNC patients with RIOM were allocated to three groups (n = 23 each): Group I (L-arginine 5 g + maltodextrin 5 g), Group II (glutamine 5 g + maltodextrin 5 g), or Group III (maltodextrin 10 g). Outcomes, assessed at weeks 2, 5, and 7 of radiotherapy, included the WHO oral mucositis scale, Pain Visual Analogue Scale (Pain-VAS), body mass index (BMI), and Oral Health Impact Profile (OHIP-14) questionnaire.
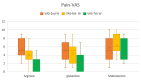
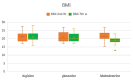
Results: By week 5, WHO scale scores differed significantly among groups (p < 0.001), with arginine and glutamine groups exhibiting lower mucositis severity than the maltodextrin group. Pain-VAS scores at weeks 5 and 7 were significantly lower in the arginine and glutamine groups compared to maltodextrin (p = 0.004 and p < 0.001, respectively). By 7th week of radiotherapy, BMI was significantly decreased in the maltodextrin group than in either the arginine (p = 0.028) or glutamine (p = 0.001) groups, indicative of treatment-mediated weight loss. In contrast, the BMI over time in the arginine (p = 0.87) and glutamine (p = 0.170) groups were almost constant. This indicates that compared to maltodextrin alone, both amino acid supplements prevented a decline in BMI during radiotherapy. OHIP-14 scores improved significantly in the arginine and glutamine groups at weeks 5 and 7 (p < 0.001), indicating better quality of life.
Conclusions: Both L-arginine and glutamine significantly reduced RIOM severity, pain, and weight loss compared to maltodextrin, while improving quality of life in patients with head and neck cancer. Although no statistically significant difference was found between the two, a higher proportion of patients receiving L-arginine achieved complete healing by week 7, suggesting a potential late advantage. These findings support the use of both amino acids as viable options for symptom management during radiotherapy.
Trial registration: ClinicalTrials.gov (NCT06764420), registered 08/01/2024.
Keywords: Head and neck cancer; L-arginine; L-glutamine; Oral mucositis; Radiotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The current study was conducted in compliance with the Helsinki Declaration for medical research involving human subjects as revised in 2013 as triple-blind, parallel arms, and randomized controlled clinical trial, with 1:1:1 allocation ratio. The study protocol was reviewed and approved by the University Research Ethics Committee (FUE.REC 2582024). All eligible individuals provided with written informed consent to participate in the current study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Anderson PM, Ramsay NKC, Shu XO, Rydholm N, Rogosheske J, Nicklow R, Skubitz KM (1998) Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant 22(4):339–344. 10.1038/sj.bmt.1701317 - PubMed
-
- Andrade ME, Trindade L, Leocádio P, Alvarez-Leite J, Reis D, Cassali G, Cardoso V (2023a) Association of Fructo-oligosaccharides and arginine improves severity of mucositis and modulate the intestinal microbiota. Probiotics Antimicrob Proteins 15. 10.1007/s12602-022-10032-8 - PubMed
-
- Andrade MER, Trindade LM, Leocádio PCL, Leite JIA, Reis D, Cassali DC, Cardoso GD, V. N (2023b) Association of Fructo-oligosaccharides and arginine improves severity of mucositis and modulate the intestinal microbiota. Probiotics Antimicrob Proteins 15(2):424–440. 10.1007/s12602-022-10032-8 - PubMed
-
- Aquino VM, Harvey AR, Garvin JH, Godder KT, Nieder ML, Adams RH, Marrow Transplant C (2005) A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant 36(7):611–616. 10.1038/sj.bmt.1705084 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

