A lentiviral vector targeting a KRAS neoepitope for cancer immunotherapy
- PMID: 40603894
- PMCID: PMC12223123
- DOI: 10.1038/s41598-025-05134-6
A lentiviral vector targeting a KRAS neoepitope for cancer immunotherapy
Abstract
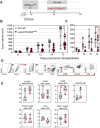
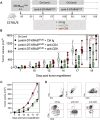
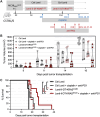
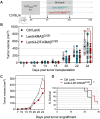
Mutated oncogenic Kirsten rat sarcoma virus (KRAS) antigen is expressed in a large variety of cancers, including pancreatic, colorectal, and pulmonary cancers. The oncogenic KRAS mutations cause malignancies and are usually ubiquitously expressed by all cells of a tumor. The KRAS amino acid substitutions at the positions 12 or 13 are among the most frequent mutations in human cancers. Here, we developed immuno-oncotherapeutic non-integrative lentiviral vectors encoding a segment encompassing KRASG12D, either alone, or associated with antigen carriers. These carriers can improve the intracellular antigen routing to major histocompatibility complex presentation machineries or provide universal helper CD4+ epitopes. Immunotherapy with one of these vectors resulted in significant immune control of tumor growth in colorectal or pulmonary preclinical cancer models, in several murine genetic backgrounds. The antitumor effect was correlated with increased proportions of intra-tumoral hematopoietic cells and notably CD8+ T cells. Although this effect was partial, it was robust, reproducible and advantageously combinable with conventional chemotherapies and immunotherapies to improve antitumor protection. Therefore, this approach shows promise as an immuno-oncotherapy against KRAS-mediated malignant transformation.
Keywords: Cancer immunotherapy; Inhibition of tumor growth; KRAS oncogenic mutations; KRASG12D; Lentiviral vectors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: PC is the founder and CSO of TheraVectys. AG, FLC, PA, KN, IF, FM, BV and AN are employees of TheraVectys. LM has a consultancy activity for TheraVectys. AG, FLC, IF, AN, CB, PC and LM are inventors of a pending patent directed to the potential of lentiviral vectors encoding KRAS mutations in immuno-oncotherapy. Other authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

