Volatile dimethyl disulphide emission from Burkholderia cepacia LS-044 suppresses metabolism and budding in caspofungin-resistant Nakaseomyces glabratus NT2
- PMID: 40603923
- PMCID: PMC12223190
- DOI: 10.1038/s41598-025-05383-5
Volatile dimethyl disulphide emission from Burkholderia cepacia LS-044 suppresses metabolism and budding in caspofungin-resistant Nakaseomyces glabratus NT2
Abstract
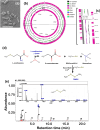
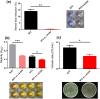
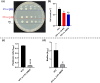
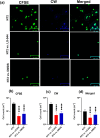
Some bacteria emit dimethyl disulphide (DMDS), one of the bioactive volatile sulphurous compounds (VSCs) of environmental and ecological significance, yet unexplored in combating drug-resistant yeasts. Here, we show the anti-budding and fungicidal activity of volatile DMDS emitted from Burkholderia cepacia LS-044 on the caspofungin-resistant yeast Nakaseomyces glabratus NT2. We identified a gene encoding L-methionine-γ-lyase (mdeA) catalysing DMDS formation in LS-044 and detected volatile DMDS as one of the VSCs (12% peak area) emitted by LS-044 through solid-phase microextraction followed by gas chromatographic-mass spectrometry. Exposure to volatiles of LS-044 resulted in a significant decline in metabolism (~ 98%), media alkalinity (~ 26%), and viable cell count (52‒95%) of NT2. Confocal microscopy of carboxyfluorescein succinimidyl ester- and calcofluor white stained cells revealed significantly high mean fluorescence in NT2 exposed to the volatiles of LS-044 (~ fivefold) and standard DMDS vapour (1.7-fold), suggesting a significant thickening of the cell wall. The surface area-to-volume ratio decreased significantly in NT2 cells exposed to volatiles of LS-044 and DMDS versus unexposed NT cells (6.5‒10.2 vs. 8.3‒13.2). DMDS exhibited a minimum inhibitory concentration of 0.5% (v/v) on NT2 cells in liquid broth dilution assay, and displayed fractional inhibitory concentration index of 0.95 with commercial antifungal clotrimazole reflecting lack of synergy or antagonism during the present combination therapy. The 26S rRNA gene sequence-based phylogeny revealed a tight phylogenetic association between NT2 and N. glabratus of environmental and clinical origins. Our study provided novel mechanistic insights into DMDS-driven bacterial antagonism on drug-resistant budding yeast N. glabratus NT2 that could be exploited in the ecological engineering and therapeutics of drug-resistant fungal infection.
Keywords: Candida glabrata; Antagonism; Fungicide; Volatile organic compounds (VOCs); Volatile sulphurous compounds (VSCs); Volatilome.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Competing interests: The authors declare no competing interests.
Figures

References
-
- Fang, N. et al. Dissipation and residues of dimethyl disulfide in tomatoes and soil under greenhouse and open field conditions. J. Environ. Sci. Health B.55, 566–573. 10.1080/03601234.2020.1740531 (2020). - PubMed
-
- Yu, J., Land, C. J., Vallad, G. E. & Boyd, N. S. Tomato tolerance and pest control following fumigation with different ratios of dimethyl disulfide and chloropicrin. Pest. Manag. Sci.75, 1416–1424. 10.1002/ps.5262 (2019). - PubMed
-
- Yu, J., Sharpe, S. M., Vallad, G. E. & Boyd, N. S. Pest control with drip-applied dimethyl disulfide and chloropicrin in plastic-mulched tomato (Solanum lycopersicum L.). Pest Manag. Sci.76, 1569–1577. 10.1002/ps.5678 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

