A paternal lactate dehydrogenase critically enhances male gametogenesis and malaria transmission
- PMID: 40603939
- PMCID: PMC12223287
- DOI: 10.1038/s41598-025-05832-1
A paternal lactate dehydrogenase critically enhances male gametogenesis and malaria transmission
Abstract
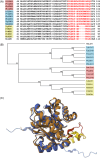
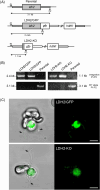
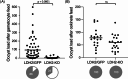
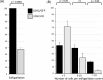
Malaria blood stage parasite development relies on glycolysis to generate ATP, which requires pyruvate to lactate conversion by an essential lactate dehydrogenase enzyme (LDH1). Conversely, parasites developing in the mosquito employ mitochondrial chemiosmosis for ATP production. The source of ATP during transition from vertebrate to insect is less clear; gametes form in the mosquito midgut lumen within minutes of gametocyte ingestion, and while female gametes possess a mitochondrion, this organelle is absent from male gametes (microgametes). Here, we investigate a second LDH enzyme (LDH2) found exclusively in male gametocytes and microgametes. Knockout of Plasmodium berghei LDH2 expression reduces the number and size of exflagellation centres and radically diminishes oocyst development in Anopheles stephensi mosquitoes. Our data indicate that LDH2 supplements LDH1 activity to facilitate the cytokinesis step of male gametogenesis, while LDH1 alone is sufficient for motility of free-swimming microgametes. Our results point to a key role for glycolytic ATP production in microgamete formation and function and identify LDH activity as a potential malaria transmission-blocking drug target.
Keywords: Gametogenesis; Glycolysis; Lactate dehydrogenase; Plasmodium; Transmission-blocking drugs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Painter, H. J., Morrisey, J. M., Mather, M. W. & Vaidya, A. B. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature446, 88–91. 10.1038/nature05572 (2007). - PubMed
-
- Royer, R. E., Deck, L. M., Campos, N. M., Hunsaker, L. A. & Vander jagt, D. L. Biologically active derivatives of gossypol: Synthesis and antimalarial activities of peri-acylated gossylic nitriles. J. Med. Chem.29, 1799–1801. 10.1021/jm00159a043 (1986). - PubMed
-
- Dunn, C. R. et al. The structure of lactate dehydrogenase from Plasmodium falciparum reveals a new target for anti-malarial design. Nat. Struct. Biol.3, 912–915. 10.1038/nsb1196-912 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

