Natural history and prognostic nomogram of untreated triple negative breast cancer based on SEER database
- PMID: 40603958
- PMCID: PMC12223081
- DOI: 10.1038/s41598-025-07114-2
Natural history and prognostic nomogram of untreated triple negative breast cancer based on SEER database
Abstract
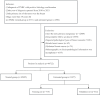
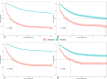
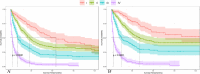
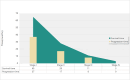
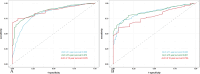
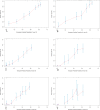
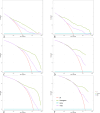
Triple-negative breast cancer (TNBC) is an aggressive subtype with a relatively poor prognosis, and its natural history without therapeutic intervention remains understudied. The purpose of this study was to investigate the natural history of untreated TNBC and construct a prognostic model utilizing data from the SEER database. Data from patients diagnosed with TNBC between 2010 and 2021 were analyzed. Median survival times for each tumor stage were calculated to estimate disease progression time (defined as the difference in median survival between consecutive stages). Cox regression was employed to identify independent prognostic factors in a training set, and a prognostic nomogram was developed based on these factors. Results showed that median survival times for untreated TNBC were 65 months (stage I), 28 months (stage II), 11 months (stage III), and 3 months (stage IV), with estimated progression times from stage I to II, II to III, and III to IV being 37 months, 17 months, and 8 months, respectively. Age, multifocal breast cancer, T stage, M stage, liver metastasis, and brain metastasis were identified as independent risk factors. The nomogram demonstrated excellent predictive performance, with a C-index of 0.795 in the training set and 0.759 in the validation set, supported by favorable calibration plots and decision curve analysis (DCA). In conclusion, untreated TNBC exhibited accelerated progression and deteriorating prognosis with increasing tumor stage, with age and tumor burden serving as key prognostic indicators, and the constructed nomogram provided a reliable tool for predicting survival in untreated TNBC patients.
Keywords: Natural history; Nomogram; Surveillance Epidemiology End results (SEER); Triple negative breast cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This study protocol was approved by the Ethics Committee of Suining Central Hospital. All information from the SEER program is available and free for public, and all patient data are deidentified, so informed consent is not required.
Figures

Similar articles
-
Nomogram for the prediction of the prognosis of patients with triple-negative invasive ductal carcinoma of breast after neoadjuvant chemotherapy.Sci Rep. 2025 Jul 1;15(1):21666. doi: 10.1038/s41598-025-05738-y. Sci Rep. 2025. PMID: 40596034 Free PMC article.
-
Establishment of prognostic model for invasive ductal carcinoma with distant metastasis within the triple-negative breast cancer: a SEER population-based study.Eur J Cancer Prev. 2025 Sep 1;34(5):392-404. doi: 10.1097/CEJ.0000000000000925. Epub 2025 Jul 30. Eur J Cancer Prev. 2025. PMID: 39724567
-
A prognostic nomogram and risk classification system of elderly patients with extraosseous plasmacytoma: a SEER database analysis.J Cancer Res Clin Oncol. 2023 Dec;149(20):17921-17931. doi: 10.1007/s00432-023-05492-6. Epub 2023 Nov 13. J Cancer Res Clin Oncol. 2023. PMID: 37955685 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74(1), 12–49 (2024). - PubMed
-
- Sung, H. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

