Barbigerone attenuates 3-nitropropionic acid-induced Huntington's disease-like neuropathology in rats via antioxidant, anti-inflammatory, and neuroprotective mechanisms
- PMID: 40603982
- PMCID: PMC12222669
- DOI: 10.1038/s41598-025-07181-5
Barbigerone attenuates 3-nitropropionic acid-induced Huntington's disease-like neuropathology in rats via antioxidant, anti-inflammatory, and neuroprotective mechanisms
Abstract
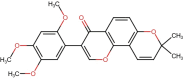
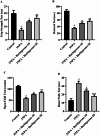
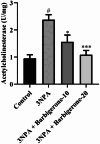
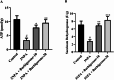
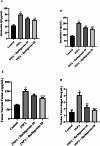
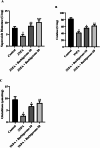
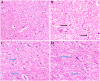
Huntington's Disease (HD), a neurodegenerative disease characterized by motor and cognitive impairments, arises from genetic mutations causing protein aggregation within the brain. The 3-Nitropropionic acid (3-NPA) rat model mimics key features of HD. This study explored the therapeutic efficacy of barbigerone, a compound with antioxidant and anti-inflammatory properties, in ameliorating 3-NPA-induced neurodegeneration and cognitive deficits in rats. Male Wistar rats were randomized into four groups: a normal control group, a 3-NPA control group, and two groups treated with different doses of barbigerone along with 3-NPA. Behavioral test, biochemical assays, and histopathological examinations were performed. Barbigerone significantly (P < 0.0001) restored motor coordination, grip strength, and mobility compared to 3-NPA-induced HD rats. Barbigerone concomitantly reduced oxidative stress by lowering malondialdehyde (MDA) and nitric oxide (NO) levels, while enhancing antioxidant enzymes such as glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT). Furthermore, treatment modulated the pro-inflammatory cytokines, suggesting a reduction in neuroinflammation. Barbigerone significantly (P < 0.0001) impacted changes in acetylcholinesterase (AChE) activity and modulated levels of key neurotransmitters, such as acetylcholine (ACh), norepinephrine (NE), serotonin (5-HT), gamma-aminobutyric acid (GABA), dopamine (DA), and glutamate (GLU). Additionally, barbigerone effectively attenuated the 3-NPA-induced elevation of caspase-3 and caspase-9, while upregulating brain-derived neurotrophic factor (BDNF), which is crucial for neuronal survival and cognitive function. Histopathological examination revealed that barbigerone significantly restored the altered striatal architecture, indicating a protective effect against neurodegeneration. Barbigerone exhibits potential as a therapeutic choice for HD, offering the possibility of alleviating motor impairments, oxidative stress, inflammation, and cognitive dysfunction.
Keywords: 3-Nitropropionic acid; Barbigerone; Cognitive function; Huntington’s disease; Motor deficits; Neuroprotective; Oxidative stress.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Coniferaldehyde reverses 3-nitropropionic acid-induced Huntington's disease pathologies via PKM2 restoration and JAK2/STAT3 inhibition.Mol Med. 2025 Jul 31;31(1):271. doi: 10.1186/s10020-025-01308-0. Mol Med. 2025. PMID: 40739177 Free PMC article.
-
6-shogaol against 3-Nitropropionic acid-induced Huntington's disease in rodents: Based on molecular docking/targeting pro-inflammatory cytokines/NF-κB-BDNF-Nrf2 pathway.PLoS One. 2024 Jul 15;19(7):e0305358. doi: 10.1371/journal.pone.0305358. eCollection 2024. PLoS One. 2024. PMID: 39008492 Free PMC article.
-
Targeting IDO1 in Huntington's Disease: Network Pharmacology and Preclinical Evidence from Coffea arabica.Neurochem Res. 2025 Aug 13;50(4):263. doi: 10.1007/s11064-025-04509-5. Neurochem Res. 2025. PMID: 40801953
-
Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models.Int J Mol Sci. 2025 Jul 21;26(14):7026. doi: 10.3390/ijms26147026. Int J Mol Sci. 2025. PMID: 40725274 Free PMC article. Review.
-
Evidence based molecular pathways, available drug targets, pre- clinical animal models and future disease modifying treatments of huntington's disease.Mol Biol Rep. 2025 Jul 25;52(1):754. doi: 10.1007/s11033-025-10852-1. Mol Biol Rep. 2025. PMID: 40711513 Review.
References
-
- Wang, L. et al. Effect of Praeruptorin C on 3-nitropropionic acid induced huntington’s disease-like symptoms in mice. Biomed. Pharmacother.86, 81–87 (2017). - PubMed
-
- Zuccato, C., Valenza, M. & Cattaneo, E. Molecular mechanisms and potential therapeutical targets in huntington’s disease. Physiol. Rev.90 (3), 905–981 (2010). - PubMed
-
- Ross, C. A. & Tabrizi, S. J. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol.10 (1), 83–98 (2011). - PubMed
-
- Chang, C.-P., Wu, C.-W. & Chern, Y. Metabolic dysregulation in Huntington’s disease: neuronal and glial perspectives. Neurobiol. Dis.2024, 106672 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

