Ionizing radiation triggers the release of mitochondrial DNA into the cytosol as a signal of mitochondrial damage
- PMID: 40603988
- PMCID: PMC12223216
- DOI: 10.1038/s41598-025-04845-0
Ionizing radiation triggers the release of mitochondrial DNA into the cytosol as a signal of mitochondrial damage
Abstract
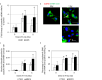
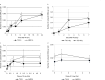
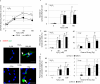
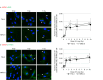
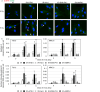
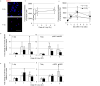
Inflammatory responses are crucial for repairing radiation-induced tissue damage. Excessive tissue remodeling in response to severe tissue injury causes chronic inflammation associated with various diseases including cardiovascular disease and cancer. Fibroblasts are major components of the stroma and play key roles in tissue remodeling. However, causes of inflammatory response activation remain unclear. This study focused on cytosolic mitochondrial DNA (mtDNA) release and its role in inflammation following irradiation. Cytosolic mtDNA leakage increased 3 h after irradiation of normal human fibroblasts and persisted for at least 7 days. H2O2 treatment of fibroblasts increased reactive oxygen species (ROS) levels, the number of cytosolic DNA per cell, and the number of senescent cells, indicating that ROS trigger cytosolic DNA release in association with cellular senescence. The cytosolic mtDNA was then recognized by the DNA sensor cyclic GMP-AMP synthase (cGAS), activating the cGAS/stimulator of interferon genes (STING) signaling pathway. DNA-PK and AMPK inhibitors prevented cytosolic mtDNA release and its colocalization with cGAS following irradiation. Cytosolic and extracellular mtDNA release was also induced in mouse upon whole-body irradiation. Our results demonstrated that mitochondrial damage signals spread throughout the body via exosomes or as cell-free DNA. Released mtDNA act as danger signals that trigger inflammation.
Keywords: Cytosolic DNA; Exosome; Mitochondrial DNA; Radiation; cGAS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 16, 582–598. 10.1038/nrc.2016.73 (2016). - PubMed
-
- Shimura, T. et al. Radiation-Induced myofibroblasts promote tumor growth via mitochondrial ROS-Activated TGFβ signaling. Mol. cancer Research: MCR. 16, 1676–1686. 10.1158/1541-7786.mcr-18-0321 (2018). - PubMed
-
- Van Linthout, S., Miteva, K. & Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovascular. Res.102, 258–269. 10.1093/cvr/cvu062 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

