Protein-bound uremic toxin clearance as biomarker of kidney tubular function in diabetic kidney disease
- PMID: 40604005
- PMCID: PMC12222703
- DOI: 10.1038/s41598-025-07248-3
Protein-bound uremic toxin clearance as biomarker of kidney tubular function in diabetic kidney disease
Abstract
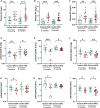
Kidney tubular damage is an important prognostic determinant in diabetic kidney disease (DKD). A vital homeostatic function of the proximal tubule is active tubular secretion of waste products via organic anion transporters (OATs), including protein-bound uremic toxins (PBUTs) that accumulate in plasma in tubular dysfunction. We here hypothesize that PBUT clearance may be a sensitive tubular function marker, and tested this in a DKD mouse model and in type 2 diabetic patients. Among the PBUTs with the highest OAT affinity (i.e., indoxyl sulfate (IS), hippuric acid (HA) and kynurenic acid (KA)), plasma concentrations were higher and urinary excretions were lower 6 and 8 months after DKD induction in mice. These parameters correlated better with tubular atrophy, f4/80 scores and tubular injury markers than conventional filtration markers. In patients, the clearance of IS, HA, KA and p-cresyl sulfate (PCS) was associated with urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1), independent of eGFR. In multiple regression analysis, additionally adjusted for relevant risk factors for tubular injury, the clearance of IS, HA and PCS remained significantly associated with urinary NGAL. In conclusion, IS, HA, KA and PCS clearance may represent a biomarker of kidney tubular function in DKD.
Keywords: Diabetic kidney disease; Hippuric acid; Indoxyl sulfate; Protein-bound uremic toxins; Tubular function marker.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent: The clinical study was registered in the Netherlands Trial Register (NTR 4439), complied with the Declaration of Helsinki and Good Clinical Practice Guidelines, and was approved by the Medical Ethics Committee of the University Medical Center Groningen, the Netherlands (METc 2014/111) (28). All participants (N = 33) provided written informed consent before any study procedure started. All animal experiments were performed with the approval of the Experimental Animal Ethics Committee of the University of Utrecht (DEC number 2009.II.11.129).
Figures

Similar articles
-
Indoxyl sulfate as a potential tubular function marker across kidney disease models.Am J Physiol Renal Physiol. 2025 Jul 1;329(1):F160-F177. doi: 10.1152/ajprenal.00107.2025. Epub 2025 Jun 11. Am J Physiol Renal Physiol. 2025. PMID: 40499558
-
Medium chain fatty acids are potent binding competitors to improve protein-bound uremic toxin clearance during hemodialysis.Kidney Int. 2025 Sep;108(3):411-426. doi: 10.1016/j.kint.2025.06.004. Epub 2025 Jun 25. Kidney Int. 2025. PMID: 40578687
-
GUT-DERIVED UREMIC TOXICITY IN LIONS (PANTHERA LEO) WITH CHRONIC KIDNEY DISEASE: A POTENTIAL THERAPEUTIC TARGET?J Zoo Wildl Med. 2025 Jun;56(2):258-271. doi: 10.1638/2024-0094. J Zoo Wildl Med. 2025. PMID: 40638166
-
Animal Models for Studying Protein-Bound Uremic Toxin Removal-A Systematic Review.Int J Mol Sci. 2023 Aug 25;24(17):13197. doi: 10.3390/ijms241713197. Int J Mol Sci. 2023. PMID: 37686004 Free PMC article.
-
Are Urinary Tubular Injury Markers Useful in Chronic Kidney Disease? A Systematic Review and Meta Analysis.PLoS One. 2016 Dec 1;11(12):e0167334. doi: 10.1371/journal.pone.0167334. eCollection 2016. PLoS One. 2016. PMID: 27907168 Free PMC article.
References
-
- Atkins, R. C. The epidemiology of chronic kidney disease. Kidney Int.67, S14–S18. 10.1111/j.1523-1755.2005.09403.x (2005). - PubMed
-
- Gupta, S., Dominguez, M. & Golestaneh, L. Diabetic Kidney Disease: An Update. Med Clin North Am107, 689–705. 10.1016/j.mcna.2023.03.004 (2023). - PubMed
-
- Rossing, P. et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int.102, 990–999. 10.1016/j.kint.2022.06.013 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- Kolff+ program (DETOX; 22OK1019)/Dutch Kidney Foundation
- CP1805 "TASKFORCE"/Dutch Kidney Foundation
- CP1805 "TASKFORCE"/Dutch Kidney Foundation
- Kolff+ program (DETOX; 22OK1019)/Dutch Kidney Foundation
- Kolff+ program (DETOX; 22OK1019)/Dutch Kidney Foundation
- KIDNEW, HORIZON-EIC-2022 Pathfinder program, grant agreement no. 101099092/European Commission
- KIDNEW, HORIZON-EIC-2022 Pathfinder program, grant agreement no. 101099092/European Commission
- Marie Skłodowska-Curie grant agreement No 813839/European Union's Horizon 2020 research and innovation programme
- Marie Skłodowska-Curie grant agreement No 813839/European Union's Horizon 2020 research and innovation programme
- KidneyX (MI-TRAM)/a public-private partnership between the American Society of Nephrology (ASN) and the US Department of Health and Human Services (HHS)
- KidneyX (MI-TRAM)/a public-private partnership between the American Society of Nephrology (ASN) and the US Department of Health and Human Services (HHS)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

