Advanced ultrasound methods to improve chronic kidney disease diagnosis
- PMID: 40604097
- PMCID: PMC12118757
- DOI: 10.1038/s44303-024-00023-5
Advanced ultrasound methods to improve chronic kidney disease diagnosis
Abstract
Chronic kidney disease (CKD) affects 850 million people worldwide and is associated with significant cardiovascular morbidity and mortality. Routine laboratory tests do not reflect early stages of microcirculatory changes and vascular rarefaction that characterise kidney fibrosis, the common endpoint of CKD. Imaging techniques that detect CKD in early stages could promote timely treatment with new drugs like SGLT2 inhibitors, thus, decreasing CKD progression and the cardiovascular disease burden. Ultrasound is the most used imaging modality in CKD, as it is non-invasive and radiation free. Initially, ultrasound imaging was applied to assess kidney macro-morphology and to rule out ureteral obstruction. The development of higher frequency probes allowed for more detailed imaging of kidney parenchyma, and advances in Doppler ultrasound provided insights into segmental arterial flow patterns including resistive indices as an indirect measure of microcirculatory impedance, elevated values of which correlated with progressive organ failure and fibrosis. Today, low-flow detection methods and matrix probes better resolve organ parenchyma and smaller vascular beds, and contrast-enhanced ultrasound allows perfusion measurement. Particularly, super-resolution ultrasound imaging, a technology currently being in clinical translation, can characterise the microcirculation morphologically and functionally in unrivalled detail. This is accompanied by rapid developments in radiomics and machine learning supporting ultrasound image acquisition and processing, as well as lesion detection and characterisation. This perspective article introduces emerging ultrasound methods for the diagnosis of CKD and discusses how the promising technical and analytical advancements can improve disease management after successful translation to clinical application.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Author SF has previously acted as a paid consultant for or received lecture fees from Stadapharm, Medice and AstraZeneca (not ultrasound related.) She is a member of the German Society for Ultrasound in Medicine (DEGUM) and a qualified ultrasound instructor “DEGUM II Internal Medicine”. FK and GS collaborate with Fujifilm Visualsonics on super-resolution ultrasound and FK additionally is an advisor of the company. FK is co-owner of the SonoMAC GmbH.
Figures


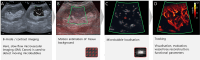
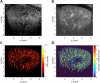

References
-
- Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004). - PubMed
-
- National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis.39, S1–266 (2002). - PubMed
-
- Miller, W. G. et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: Practical guidance for clinical laboratories. Clin. Chem.68, 511–520 (2022). - PubMed
-
- Klinkhammer, B. M. et al. Current kidney function parameters overestimate kidney tissue repair in reversible experimental kidney disease. Kidney Int.102, 307–320 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
