Determination of the median effective dose of sufentanil combined with remimazolam in inhibiting tracheal intubation response
- PMID: 40604110
- PMCID: PMC12222887
- DOI: 10.1038/s41598-025-08907-1
Determination of the median effective dose of sufentanil combined with remimazolam in inhibiting tracheal intubation response
Abstract
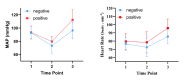
To determine the median effective dose (ED50) and 95% effective dose (ED95) of sufentanil combined with remimazolam for inhibiting the tracheal intubation response in patients undergoing general anesthesia and to evaluate the hemodynamic stability and adverse events associated with this drug combination. This prospective dose-finding study used Dixon's up-and-down sequential allocation method. A total of 36 patients undergoing general anesthesia surgery between April 2024 and June 2024 were enrolled. Patients were administered remimazolam for induction, followed by sufentanil at an initial dose of 0.4 µg/kg, with subsequent doses adjusted based on the presence or absence of an intubation response. The primary outcome was the ED50 of sufentanil combined with remimazolam, and the secondary outcomes included patient baseline characteristics, hemodynamic parameters, and adverse events. The ED50 and ED95 of sufentanil for inhibiting tracheal intubation response were 0.374 µg/kg (95% CI: 0.342-0.402 µg/kg) and 0.436 µg/kg (95% CI: 0.406-0.586 µg/kg), respectively. Patients with a positive tracheal intubation response had significantly higher heart rates and mean arterial pressures and a higher incidence of hypertension. The ED50 and ED95 of sufentanil combined with remimazolam for inhibiting the tracheal intubation response in patients undergoing general anesthesia were 0.374 and 0.436 µg/kg, respectively. This study provides valuable insights into the dosing of these drugs for effective anesthesia induction and hemodynamic control during tracheal intubation.
Keywords: Anesthesia induction; Median effective dose (ED50); Remimazolam; Sufentanil; Tracheal intubation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Nanchong Central Hospital (2023, trial (061) No.). Each patient signed an informed consent procedure before surgery. We confirm our study complies with the Declaration of Helsinki.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous