Aldehydic load as an objective imaging biomarker of mild traumatic brain injury
- PMID: 40604170
- PMCID: PMC12202811
- DOI: 10.1038/s44303-025-00096-w
Aldehydic load as an objective imaging biomarker of mild traumatic brain injury
Erratum in
-
Author Correction: Aldehydic load as an objective imaging biomarker of mild traumatic brain injury.Npj Imaging. 2025 Oct 6;3(1):47. doi: 10.1038/s44303-025-00114-x. Npj Imaging. 2025. PMID: 41053357 Free PMC article. No abstract available.
Abstract
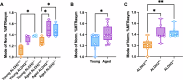
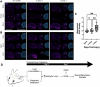
Mild traumatic brain injury (mTBI) is neurological impairment induced by biomechanical forces without structural brain damage, currently without an objective diagnostic tool. Downstream injury stems from oxidative damage leading to the production of neurotoxic aldehydes. A collagen-based 3D corticomimetic in vitro model of concussion was developed, confirming aldehyde production following impact. Total aldehyde levels were mapped in vivo following mTBI using a novel CEST-MRI contrast agent, ProxyNA3, in a new model of closed-head, awake, single-impact concussion in aged and young mice with aldehyde dehydrogenase 2 (ALDH2) deficiency. ProxyNA3-MRI was performed before impact, and on days two- and seven- post-impact. MRI signal enhancement significantly increased at two days post-injury prior to astrocyte activation at seven days post-injury. The data suggest that advanced age and ALDH2 deficiency contribute to increased aldehydic load following mTBI. Overall, ProxyNA3 was capable of mapping concussion-associated aldehydes, supporting its application as an objective diagnostic tool for concussion.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.J.S., A.K., and M.S. declare no Competing Financial interests but the following Competing Non-Financial Interests: the authors have filed a patent (U.S. 11,696,960) regarding the use of N-aminoanthranilic acids for aldehyde imaging. A.J.S. is an Associate Editor for npj Imaging, but was not involved in the editorial review of, or decision to publish this article. All other authors declare no competing interests.
Figures




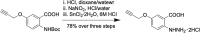
References
-
- Cassidy, J. D. et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 28–60. 10.1080/16501960410023732 (2004). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
