Prenylated chalcone analog-mediated Inhibition of Toxoplasma gondii growth in human trophoblast cell line and villous explants
- PMID: 40604186
- PMCID: PMC12223089
- DOI: 10.1038/s41598-025-08764-y
Prenylated chalcone analog-mediated Inhibition of Toxoplasma gondii growth in human trophoblast cell line and villous explants
Abstract
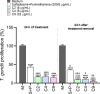
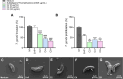
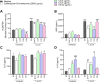
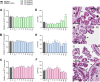
Congenital toxoplasmosis is a significant public health issue caused by the transplacental passage of Toxoplasma gondii to the embryo/fetus. The standard treatment involves a combination of sulfadiazine and pyrimethamine, drugs often associated with adverse effects and high toxicity. The current study aimed to investigate the potential of prenylated chalcones (C2, C4 and C9) in controlling T. gondii infection in human trophoblast cells (BeWo) and human placental explants. As results, non-cytotoxic doses of C2, C4 and C9 impaired parasite invasion and subsequent intracellular proliferation in BeWo cells. Scanning and transmission electron microscopies evidenced the direct effect of chalcones on tachyzoites, which presented irregular rough surface, membrane with hole-like structures, torsion and shape substantial changes after pretreatment. C4 and, especially C9, caused notable ultrastructural damages due to the formation of vacuole-like structures in the parasite cytoplasm and surrounding the parasitophorous vacuole. Additionally, chalcones modulated the cytokine profile by increasing IL-8 and downmodulating MIF and ROS levels in BeWo cells and downregulating TNF-α release in villous explants. These findings highlight C2, C4, and C9 as promising candidates for the development of alternative therapies to prevent congenital toxoplasmosis, as well as chalcones as a valuable scaffold for the design of new anti-T. gondii agents.
Keywords: Toxoplasma gondii; Chalcones; Flavonoid; Placenta; Treatment; Trophoblast.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests and no competing financial interests.
Figures



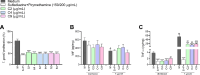
References
-
- Montoya, J. G., Liesenfeld, O. & Toxoplasmosis Lancet 363, 1965– (1976). 10.1016/s0140-6736(04)16412-x (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

