Effectiveness of symptom monitoring on electronic patient-reported outcomes (ePROs) among patients with lung cancer: a systematic review and meta-analysis
- PMID: 40604235
- PMCID: PMC12223247
- DOI: 10.1038/s41746-025-01812-x
Effectiveness of symptom monitoring on electronic patient-reported outcomes (ePROs) among patients with lung cancer: a systematic review and meta-analysis
Abstract
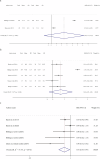
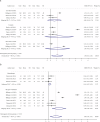
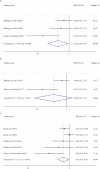
Symptom monitoring using electronic patient-reported outcomes (ePROs) has demonstrated benefits for patients with cancer, yet the systematic effects for lung cancer remains unknown. This study performed a literature search in Medline, Embase, Cochrane library, CINAHL and APA PsyInfo before April 23rd, 2025, and identified 5755 papers. 18 (0.31%) papers from 11 studies conducting symptom monitoring on ePROs and sending alerts of severe symptoms were included. The meta-analysis showed significant improvement in health-related quality of life (SMD = 2.44, P < 0.001) among patients with lung cancer, with an intervention duration of less than 6 months, 6 months and more than 6 months. When excluding studies that sent alerts to patients themselves, overall survival for lung cancer patients was significantly prolonged (HR = 0.54, 95% CI [0.22, 1.31], P = 0.031). Symptom burden, physical functioning, and healthcare service utilization was also advantaged, but implementation process and cost-effectiveness data was insufficient (Trial registration: CRD420251000397).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Exercise interventions on health-related quality of life for people with cancer during active treatment.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. Cochrane Database Syst Rev. 2012. PMID: 22895974 Free PMC article.
-
Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011425. doi: 10.1002/14651858.CD011425.pub2. Cochrane Database Syst Rev. 2017. PMID: 28535331 Free PMC article.
-
Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis.Health Technol Assess. 2012;16(38):1-205, iii-v. doi: 10.3310/hta16380. Health Technol Assess. 2012. PMID: 23046909
References
-
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin.71, 209–249, 10.3322/caac.21660 (2021). - PubMed
-
- Leiter, A., Veluswamy, R. R. & Wisnivesky, J. P. The global burden of lung cancer: current status and future trends. Nat. Rev. Clin. Oncol.20, 624–639, 10.1038/s41571-023-00798-3 (2023). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

