The effect of high-sugar feeding on rodent metabolic phenotype: a systematic review and meta-analysis
- PMID: 40604308
- PMCID: PMC12118738
- DOI: 10.1038/s44324-024-00043-0
The effect of high-sugar feeding on rodent metabolic phenotype: a systematic review and meta-analysis
Abstract
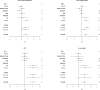
Dietary sugar consumption has been linked to increased cardiometabolic disease risk, although it is unclear if this is independent of increases in body weight and adiposity. Additionally, many preclinical animal studies provide liquid sugar which more readily leads to excess consumption and weight gain, confounding any outcomes driven by high-sugar intake alone. To gain clarity on this, we conducted a systematic review and meta-analysis exclusively investigating the effect of isocaloric high-sugar, low-fat solid diet formulations containing fructose or sucrose, on cardiometabolic health in rodents. Overall, we found strong evidence that fructose and sucrose have effects on metabolic health, independent of body weight gain. High-sugar feeding, with fructose in particular, altered liver phenotype; ALT (d = 1.08; 0.66, 1.5), triglyceride content (d = 0.52; 0.25, 0.78), cholesterol (d = 0.59; 0.16, 1.03) and liver mass (d = 0.93; 0.37, 1.48), and glucose tolerance; fasting glucose (d = 0.60; 0.18, 1.01) and fasting insulin (d = 0.42; 0.07, 0.77) but not body weight or energy intake. Our review also highlights the lack of data reported on adiposity and in female rodents. This is the first meta-analysis to synthesise all current rodent solid diet high-sugar studies, while adjusting them for confounders (fat content, time spent on diet and age started on diet) and suggests that high-sugar dietary intake and composition alters metabolic health of mice regardless of weight gain.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
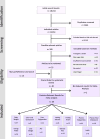
Figures



References
LinkOut - more resources
Full Text Sources
