Incretin-based therapies for the treatment of obesity-related diseases
- PMID: 40604322
- PMCID: PMC12118674
- DOI: 10.1038/s44324-024-00030-5
Incretin-based therapies for the treatment of obesity-related diseases
Abstract
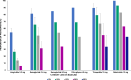
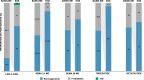
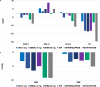
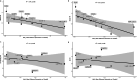
Obesity-related disability-adjusted life years (DALYs) are expected to increase by approximately 40% from 2020 to 2030. DALYs and mortality related to obesity are the consequence of multiple comorbidities such as cardiovascular (i.e., heart failure) and metabolic diseases (i.e. type 2 diabetes [T2D], metabolic dysfunction-associated steatotic liver disease [MASLD]). Lifestyle interventions represent the foundation of obesity treatment, yet an escalation to pharmacological and/or surgical interventions is often needed. Liraglutide, semaglutide and tirzepatide are incretin-based therapies currently approved by FDA for the management of obesity, while triple GIPR/GCGR/GLP-1R agonist retatrutide (LY3437943), the cagrilintide/semaglutide (CagriSema) 2.4 mg combination, high-dose oral semaglutide, and oral orforglipron are in advanced stages of development. Incretin-based therapies have been associated with a body weight (BW) reduction of ≥5% in at least half of patients in most randomized controlled trials (RCT) and real-world studies (RWS). Semaglutide and tirzepatide have also displayed a mean 60-69% 10-years relative risk reduction of T2D development. In line with evidence accrued in patients with T2D, incretin-based therapies produced a favorable effect on traditional cardiovascular risk factors, such as lipids and blood pressure, and even reduced the risk of major cardiovascular events and heart failure-related events in individuals with obesity, as recently demonstrated for the first time in the SELECT trial with semaglutide 2.4 mg once-weekly. Moreover, incretin-based therapies have also been proven beneficial on obesity-related comorbidities, such as knee osteoarthritis (KOA), obstructive sleep apnea (OSA) syndrome, and MASLD. Further research is needed to improve our understanding of their effects on obesity-related comorbidities and the underlying mechanism, whether involving direct effects on target tissues or mediated by improvement in BW, glucose levels and other CV risk factors.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: A.C.: AstraZeneca, Eli Lilly, Novo Nordisk, Roche Diagnostics, Sanofi Aventis. F.G.: AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Lifescan, Medimmune, Merck Sharp & Dohme, Medtronic, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis. G.P.S.: Amgen, Amryt, Eli Lilly, Farmitalia, Novo Nordisk, Sanofi; I.C.: Eli Lilly, Novo Nordisk; S.P.: no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
