Genetically determined Alzheimer's disease research advances: The Down Syndrome & Autosomal Dominant Alzheimer's Disease 2024 Conference
- PMID: 40604347
- PMCID: PMC12221810
- DOI: 10.1002/alz.70309
Genetically determined Alzheimer's disease research advances: The Down Syndrome & Autosomal Dominant Alzheimer's Disease 2024 Conference
Abstract
Introduction: The Down syndrome-associated Alzheimer's disease (DSAD) autosomal dominant Alzheimer's disease (ADAD) 2024 Conference in Barcelona, convened under an Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART) grant through the Down syndrome and Alzheimer's disease (AD) Professional Interest Area (PIA), brought together global researchers to foster collaboration and knowledge exchange between the fields of DSAD and ADAD.
Methods: This article provides a synthesis review of the conference proceedings, summarizing key discussions on biomarkers, natural history models, clinical trials, and ethical considerations in anti-amyloid therapies.
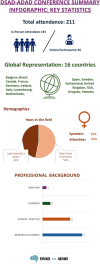
Results: A total of 211 attendees from 16 countries joined the meeting. Global researchers presented on disease mechanisms, therapeutic developments, and patient care strategies. Discussions focused on challenges and opportunities unique to DSAD and ADAD. Experts emphasized the urgent need for tailored clinical trials for ADAD and DSAD and debated the safety and efficacy of anti-amyloid treatments. Ethical considerations highlighted equitable access to therapies and the crucial role of patient and caregiver involvement.
Discussion: The conference highlighted the importance of inclusive research and collaboration across the genetic forms of AD.
Highlights: Biomarker research and natural history models developed in Down syndrome-associated Alzheimer's disease (DSAD) and autosomal dominant Alzheimer's disease (ADAD) enable the prediction of disease progression not only for DSAD and ADAD, but also for sporadic Alzheimer's disease (AD). -Collaboration and knowledge exchange among researchers across these genetic forms of AD will accelerate our understanding of the pathophysiology and advance preventive trials in DSAD and ADAD. -Tailored clinical trials for DSAD are urgently needed to address specific safety and efficacy concerns. -Inclusive research practices are crucial for advancing treatments and understanding of DSAD and ADAD.
Keywords: Autosomal dominant Alzheimer's disease; Biomarkers; Clinical trials; Down syndrome; Neuroimaging; Pathophysiology.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Instituto de Salut Carlos III supported L.M.B. and N.F. and co‐funded by the European Union through the Río Hortega Fellowship “CM23/00291” (L.M.B.) and Juan Rodés grant JR22/00014 (N.F.). N.S.R. acknowledges support from the UK Dementia Research Institute at UCL through UK DRI Ltd, principally funded by the UK Medical Research Council, the UK NIHR UCLH Biomedical Research Centre, and the Dominantly Inherited Alzheimer Network, funded by the National Institute on Aging. L.M. is funded by grants from the “Fondation Jérôme Lejeune”, the “Agence Nationale de la Recherche (ANR)” (DYRK‐DOWN, TRANSBIOROYAL and KINHIB‐DIAB projects), France 2030 (i‐Nov vague 9, Leucettinib‐21 project), and Bpifrance (EUROSTARS, T2DiaCURE project), the European Union's Horizon 2020 research and innovation program (GO‐DS21 project) and the European Innovation Council (EIC) Accelerator Program (DOWN‐AUTONOMY project, 190138295). Alzheimer's Association ISTAART Conference Support Award. H.M.S. is a full‐time employee of the Alzheimer's Association, and their spouse is an employee at Abbott Labs in an unrelated field. L.V.A. was supported by Instituto de Salud Carlos III through the Sara Borrell Postdoctoral Fellowship “CD23/00235”. M.C.I. acknowledges support from the Alzheimer's Association and Global Brain Health Institute (GBHI_ALZ‐18‐543740), the Jérôme Lejeune Foundation (#1913 Cycle 2019B), the Societat Catalana de Neurologia (Premi Beca Fundació SCN 2020). I.R.B. was supported by Instituto de Salud Carlos III through the Río Hortega Fellowship “CM22/00052” and co‐funded by the European Union. J.E.A.I. was supported by Instituto de Salud Carlos III through the Río Hortega Fellowship “CM22/00219” and co‐funded by the European Union. J.A. was supported by Instituto de Salud Carlos III through the Río Hortega Fellowship “CM21/00243” and co‐funded by the European Union. L.M.B. was supported by Instituto de Salud Carlos III through the Río Hortega Fellowship “CM23/00291” and co‐funded by the European Union. A.B. acknowledges support from Instituto de Salud Carlos III through the Miguel Servet grant “CP20/00038” and co‐funded by the European Union, and the Alzheimer's Association “AARG‐22‐923680.” L.D.H.S. acknowledges support from Instituto de Salud Carlos III through the Miguel Servet grant CP24/00112 and co‐funded by the European Union, and the the Jérôme Lejeune Foundation (2326 ‐ GRT‐2024A). The conference was funded by grants from the Alzheimer's Association through the International Society to Advance Alzheimer's Research and Treatment (ISTAART) Grant Program for Conferences and Convenings (IGPCC) and the Pasqual Maragall Foundation, with the support of the pharmaceutical industry. J.F. reported receiving personal fees for service on the advisory boards, adjudication committees, or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Novo Nordisk, Perha, Roche, Zambón, and outside the submitted work. D.A., A.L., and J.F. reports holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to ADx, EPI8382175.0). S.G. reported receiving personal fees for service on the advisory boards, speaker honoraria, or educational activities from Esteve, Indorsia, and Biojen. MCI reported receiving personal fees for service on the advisory boards, speaker honoraria, or educational activities from Esteve, Lilly, Neuraxpharm, Adium, and Roche. Author disclosures are available in the Supporting Information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- GBHI_ALZ-18-543740/Global Brain Health Institute
- AG/NIA NIH HHS/United States
- UK Dementia Research Institute
- Fundación Pasqual Maragall
- AARG-22-923680/ALZ/Alzheimer's Association/United States
- DYRK-DOWN/Agence Nationale de la Recherche
- 2326 - GRT-2024A/Fondation Jérôme Lejeune
- JR22/00014/Instituto de Salud Carlos III
- CD23/00235/Instituto de Salud Carlos III
- CM22/00052/Instituto de Salud Carlos III
- CM22/00219/Instituto de Salud Carlos III
- CM21/00243/Instituto de Salud Carlos III
- CP20/00038/Instituto de Salud Carlos III
- CP24/00112/Instituto de Salud Carlos III
- CM23/00291/Instituto de Salud Carlos III
- Ministerio de Ciencias Innovación y Universidades
- Fondos FEDER
- UK Medical Research Council, UK NIHR UCLH Biomedical Research Centre, Dominantly Inherited Alzheimer Network
- GO-DS21/Agence Nationale de la Recherche (ANR), France 2030, Bpifrance, European Union's Horizon 2020 research and innovation program
- 190138295/European Innovation Council (EIC), Accelerator Program
- Alzheimer's Association ISTAART Conference Support Award
- Fundación Catalana del Síndrome de Down (FCSD)
LinkOut - more resources
Full Text Sources
Medical

