Feasibility, acceptability and effectiveness of asthma education delivered by community health workers in improving asthma control in children and adolescents: a protocol for a cluster-randomized trial in Uganda
- PMID: 40604619
- PMCID: PMC12220509
- DOI: 10.1186/s12887-025-05843-x
Feasibility, acceptability and effectiveness of asthma education delivered by community health workers in improving asthma control in children and adolescents: a protocol for a cluster-randomized trial in Uganda
Abstract
Background: Asthma control is a major challenge particularly in low and middle-income countries where access to care is still poor. Asthma education is a cornerstone in self-management and achieving asthma symptom control. However, there is limited human resources to offer asthma education in low-resources settings. This study will investigate the feasibility, acceptability and effectiveness of community health worker (CHW)-led asthma education in improving asthma control among children and adolescents in Uganda.
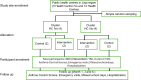
Methods: The study will employ a cluster-randomized trial (CRT) study design. It will be conducted in 8 primary care health facilities (HFs) in Jinja region, Eastern Uganda. The HFs will be randomly assigned to the control and intervention arms in the ratio 1:1. The sample size will be 300 children (150 per arm), and asthma control, emergency care visits and hospitalizations will be assessed at baseline, month 1,3 and 6. Asthma education will be provided by CHWs known as Village Health Teams (VHTs) at each visit. Data on direct and indirect costs of asthma education will be collected prospectively. Focus Group Discussions (FGDs) will be conducted among VHTs and caregivers of participating children at the intervention sites to assess the feasibility and acceptability of the intervention. To estimate the mean difference in asthma control scores between the intervention and control arms, adjusted for baseline scores and facility-level clustering, we shall use a random-effects linear regression model and intention-to-treat analysis. The primary endpoint will be asthma control scores. Cost-effectiveness will be assessed by computing the Incremental Cost-Effectiveness Ratio. Thematic content analysis will be used to analyze data from the FGDs.
Discussion: It is anticipated that the study will provide evidence about the role of community health workers in supporting the care of patients with asthma and achieving symptom control, through providing health education. The data on feasibility, acceptability and cost-effectiveness will be critical in informing scale up plans.
Trial registration number: ISRCTN16018011. Date: 25/05/2024.
Keywords: Asthma; Children adolescents; Community health workers; Education.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval for this study (Version 2.0 dated 14th March 2024) was obtained from Makerere University School of Public Health Research and Ethics Committee (SPH-2024-552), Uganda National Council for Science and Technology (HS4136ES) and the Queen Mary University of London Research Ethics Committee in the UK (QME24.0514). Approval for any protocol amendments will be sought from Makerere University School of Public Health Research and Ethics Committee. Notification of any amendments will be made to the Uganda National Council for Science and Technology and Queen Mary University of London Research Ethics Committee. All participants will provide informed consent to participate in the study before taking part in any study related activities/ procedures. Children aged 8 years and above will provide assent, in addition to consent by their parents/caregivers (supplementary file 2). Each participant will have a study number that will be used as the identifier. Only anonymised data will be collected on the questionnaires. All the information will be kept confidential. The identifiable data will only be accessed by the research team. Data protection and sharing will adhere to the Data Protection and Privacy Act 2019 of the Republic of Uganda. The trial activities are overseen by a 8-member Trial Steering Committee (TSC) comprising an independent chair, 2 independent members, a lay member, a representative from the funder (observer), representative from Makerere University where the study is anchored (observer) and one policymaker’s representative from the Ministry of Health. The study principal investigator and co-principal investigator are also members of the TSC. The TSC meetings will be held at least every 6 months to review study progress. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
References
-
- The Global Asthma Report. 2022. Contract No.: S1–102.
-
- Asthma GIf. Global Strategy for Asthma Management and Prevention. 2024.
-
- Oyenuga VO, Mosler G, Addo-Yobo E, Adeyeye OO, Arhin B, Fortune F, et al. Asthma symptoms, severity, and control with and without a clinical diagnosis of asthma in early adolescence in sub-Saharan africa: a multi-country, school-based, cross-sectional study. Lancet Child Adolesc Health. 2024;8(12):859–71. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
- MR/X031713/1/UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO)
LinkOut - more resources
Full Text Sources
Medical
Research Materials