Metabolic evaluation and disease characteristics of urolithiasis in children from 2020 to 2024: a retrospective single-center study at the national children's medical center
- PMID: 40604654
- PMCID: PMC12219921
- DOI: 10.1186/s12887-025-05773-8
Metabolic evaluation and disease characteristics of urolithiasis in children from 2020 to 2024: a retrospective single-center study at the national children's medical center
Abstract
Objective: To analyze the composition and disease characteristics of pediatric urolithiasis in a single center, providing a scientific basis for clinical diagnosis, treatment, and prevention.
Methods: This study included 266 pediatric patients with urolithiasis admitted from September 2020 to September 2024. Composition analysis was conducted on 174 stone samples using infrared spectroscopy, and 24-h urinary metabolic evaluations were performed on 124 patients. Additionally, genetic testing was conducted on 18 patients using the GenCap panel to explore the role of genetic factors in stone formation.
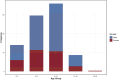
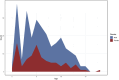
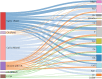
Results: Calcium oxalate stones were the most common type, accounting for 63.2% of all stones, followed by calcium phosphate and carbonate apatite stones. The high-incidence age range for stones was 6-12 years, with a male-to-female ratio of 1.86:1. The 24-h urinary metabolic analysis revealed that urinary components such as calcium, oxalate, and uric acid were closely related to stone formation. Genetic testing identified multiple different genes associated with stone formation, further confirming the importance of genetic factors.
Conclusion: The formation of pediatric urolithiasis is influenced by various factors, including metabolic abnormalities, urinary components, and genetic factors. Understanding these influencing factors contributes to the development of personalized prevention and treatment plans, reducing the recurrence rate and complications of stones.
Keywords: Disease characteristics; Genetic testing; Pediatric urolithiasis; Stone composition analysis; Urinary metabolic evaluation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Ethics Committee of the Affiliated Children's Hospital of Zhejiang University School of Medicine (Approval Number: 2024-IRB-0108-P-01). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Velásquez-Forero F, Esparza M, Salas A, et al. Risk factors evaluation for urolithiasis among children. Bol Med Hosp Infant Mex. 2016;73(4):228–36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

