The impact of the Change4Life Food Scanner app on children's diets and parental psychological outcomes: a randomised pilot and feasibility study
- PMID: 40604685
- PMCID: PMC12220540
- DOI: 10.1186/s12889-025-23400-0
The impact of the Change4Life Food Scanner app on children's diets and parental psychological outcomes: a randomised pilot and feasibility study
Abstract
Background: The Change4Life Food Scanner app raises awareness of the nutritional content of barcode-scanned packaged food through a variety of visual displays. This study investigated (1) the feasibility and acceptability of evaluating the effectiveness of the Food Scanner app in reducing children's energy (kcal) and sugar (g) intake over a 3-month period, (2) app engagement and (3) the app's impact on psychological outcomes.
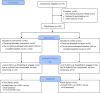
Methods: Adopting a non-blinded parallel trial design, 126 parents of 4-11 year olds were randomly assigned (1:1) through block randomisation sequences into a 3-month intervention consisting of exposure to the Food Scanner app (version 1.6; [n = 62]) or no intervention (n = 64). Intervention participants were encouraged to use the app for healthier food choices when shopping. Participants completed baseline and 3-month follow-up (3MFU) measures of child dietary intake, psychological, and health economic outcomes. Dietary intake was also assessed at 1-month. The intervention arm additionally completed fortnightly app engagement measures and all participants provided feasibility feedback at 3MFU. Mixed model Analysis of Variance and independent t-tests of mean differences assessed changes in dietary intake. Descriptive analyses were conducted for all other measures. Ethical approval was obtained by the University of Sheffield Research Ethics Committee (026380).
Results: The study was completed by 64 (51%) of 126 participants (29 [45%] in the intervention group and 35 [55%] in the control group). Most participants (> 80%) found the study acceptable, whilst 68% of intervention participants would recommend the app to others. There was a mean difference in daily energy (kcal) intake of 18 (95% CI: -180; 217) at 3MFU, and a mean difference of 10g in sugar intake (95% CI: -3; 23), between conditions, with a greater reduction within the control condition. Average app engagement declined over the study, from 14.1 min (± 14.7) in week 2 to 6.8 min (± 11.6) in week 12. Minor differences in psychological outcomes were observed between conditions.
Conclusions: Despite high attrition, study procedures were deemed feasible. Low app engagement and usage barriers may have impacted app acceptability and related outcomes. Recommendations are provided for future app development and full-scale trial design.
Trial registration: ISRCTN12169303; 12th May 2025. Retrospectively registered.
Keywords: App engagement; Behaviour change; Childhood obesity prevention; Diet; Digital intervention; Energy intake; Feasibility study; MHealth; Mobile applications; Sugars.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the declaration of Helsinki and local governance requirements. Ethical approval was obtained from the University of Sheffield Research Ethics Committee (026380). Initial approval was granted on August 2019, with further amendments placed in October 2019, December 2019 and April 2020. The nature of amendments included expanding recruitment methods and inclusion of additional survey questions. Informed and written consent was obtained from all participants before participation in the study. Participant information sheets and consent forms were accessed and completed online by parents. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Beverley B, David C, Kerry S J, Polly P, Caireen R, Toni S, et al. National Diet and Nutrition Survey Rolling programme Years 9 to 11 (2016/2017 to 2018/2019)-a survey carried out on behalf of Public Health England and the Food Standards Agency. 2020.
-
- Public Health England. Children consume more than a year’s worth of sugar in 6 months. https://www.gov.uk/government/news/children-consume-more-than-a-years-wo... (2018). Accessed 13 May 2025.
-
- NHS Digital. Statistics on Obesity, Physical Activity and Diet, England, 2020. https://digital.nhs.uk/data-and-information/publications/statistical/sta... (2020). Accessed 13 May 2025.
-
- Public Health England. Government recommendations for energy and nutrients for males and females aged 1 – 18 years and 19+ years. https://assets.publishing.service.gov.uk/media/5a749fece5274a44083b82d8/... (2016). Accessed 13 May 2025.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

