Harnessing animal model and computational experiments to discover antidiabetic compounds in Ficus racemosa
- PMID: 40604816
- PMCID: PMC12220644
- DOI: 10.1186/s12906-025-04845-7
Harnessing animal model and computational experiments to discover antidiabetic compounds in Ficus racemosa
Abstract
Background: Diabetes mellitus (DM) is a major health concern caused by poor blood sugar regulation. Despite oral hypoglycemic medications, diabetes and its complications remain clinically serious. Using animal models and in silico research, the antidiabetic potential of Ficus racemosa (F. racemosa) has been assessed in this study.
Materials and methods: The methanol extract of F. racemosa fruits was prepared using suitable methods. After injecting Alloxan (150 mg/kg) into Swiss Albino mice to cause diabetes, both diabetic and non-diabetic animals underwent OGTT and acute toxicity testing. The antihyperglycemic action was assessed by administering oral doses of 300 and 500 mg/kg of the methanol extract of F. racemosa fruit, as well as 5 mg/kg of glibenclamide. Subsequently, in silico techniques such as ADMET profiling, molecular docking, and simulations were employed.
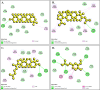
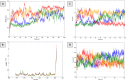
Results: The findings from this study suggest that mice have tolerated doses under 3000 mg/kg without death or side effects. In mice model, both doses of F. racemosa extracts effectively reduced blood glucose (BGL) after 7 days of oral administration. Molecular docking and simulations demonstrated that the SIRT1 receptor had a greater affinity for friedelin, lupeol acetate, gluanol, and ferulic acid. The molecular dynamics demonstrated that all the compounds are stable to the receptors, as revealed by RMSD, RMSF, Rg and SASA parameters.
Conclusion: This study found that F. racemosa fruit extract significantly reduced hyperglycemia. Furthermore, four compounds may significantly contribute to the treatment of diabetes by reducing blood glucose levels. Thus, the findings of the current study may strengthen future research in the identification of antidiabetic compounds.
Keywords: Ficus racemosa; Diabetes mellitus; Diabetic treatment; Mice model; OGTT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animals were maintained in a pathogen-free environment. The animal care and use protocols were approved by the Institutional Ethical Review Board (IRB) of National Institute of Biotechnology under ethical approval no: NIB/IRB/2023/BID-02. Additionally, permission for plant sample collection was obtained from the Deputy Commissioner of Rangamati, Bangladesh (Reference No.: 39.06.2672.013.14.002.22). All procedures were performed in compliance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


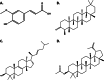


References
-
- American Diabetes Association, “2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021,”. Diabetes Care. 2021;44(Supplement_1):S15–S33. 10.2337/dc21-S002. - PubMed
-
- National Institute of Diabetes and Digestive and Kidney Diseases, “‘Diabetes Diet, Eating, & Physical Activity’ .” Accessed: Oct. 12, 2023. [Online]. Available: https://www.niddk.nih.gov/health-information/diabetes/overview/diet-eati...
-
- M. J. B. van Baar, C. C. van Ruiten, M. H. A. Muskiet, L. van Bloemendaal, R. G. IJzerman, and D. H. van Raalte, “SGLT2 Inhibitors in Combination Therapy: From Mechanisms to Clinical Considerations in Type 2 Diabetes Management,”. Diabetes Care. 2018;41(8)1543–1556. 10.2337/dc18-0588. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

