STK3 is a transcriptional target of YAP1 and a hub component in the crosstalk between Hippo and Wnt signaling pathways during gastric carcinogenesis
- PMID: 40604818
- PMCID: PMC12220525
- DOI: 10.1186/s12943-025-02391-x
STK3 is a transcriptional target of YAP1 and a hub component in the crosstalk between Hippo and Wnt signaling pathways during gastric carcinogenesis
Abstract
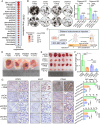
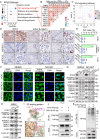
Background: Serine/threonine kinase 3 (STK3) is recognized as a key regulator in Hippo pathway and a tumor-suppressing gene in various cancer types. However, its non-canonical role has been gradually revealed in cancer development.
Methods: Our objective is to elucidate the upregulation pattern and molecular mechanisms of STK3 in advancing gastric cancer (GC) progression. The regulation of YAP1 on STK3 was assessed through a combination of bulk and single-cell RNA-sequencing, Western blot, ChIP-qPCR, gene knockout mouse models, and functional rescue assays. The oncogenic roles of STK3 were confirmed through subcutaneous xenograft formation models and functional assays including spheroid formation and organoid growth. The phosphorylated target of STK3 was revealed by co-immunoprecipitation and in vitro kinase assays. STK3-targeted drugs were screened out by molecular docking and cellular thermal shift assay (CETSA).
Results: Reduction of YAP1 significantly impaired STK3 expression at both mRNA and protein levels, and deletion of STK3 partially attenuated the oncogenic activity of YAP1. Notably, MNNG-induced tumors in Yap1-/-Taz-/- mice exhibited decreased STK3 expression. Knockdown of STK3 led to reduced expression of stemness markers and xenograft growth, while sensitizing GC organoids and xenografts to 5-fluorouracil treatment. Mechanistically, the direct interaction between STK3 and GSK-3β promoted GSK-3β phosphorylation and β-catenin nuclear accumulation, and thus the activation of Wnt signaling. Furthermore, aminopterin demonstrates as a promising STK3-targeted small molecule with remarkable effectiveness in inhibiting GC cell malignance and xenograft growth.
Conclusions: STK3 was identified as a transcriptional target of YAP1, leading to enhanced DNA repair ability and stemness acquisition during GC progression by activating Wnt/β-catenin activity through GSK-3β degradation. Moreover, STK3-targeted therapy offered a novel approach to concur acquired chemo-resistance in GC patients.
Keywords: Gastric cancer; STK3; Wnt signaling; YAP1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The use of human samples was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (CREC Ref. No.: 2022-060). A waiver of consent form was granted by the Ethics Committee. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
KAP1 promotes gastric adenocarcinoma progression by activating Hippo/YAP1 signaling via binding to HNRNPAB.Cancer Lett. 2025 Jul 1;621:217695. doi: 10.1016/j.canlet.2025.217695. Epub 2025 Apr 4. Cancer Lett. 2025. PMID: 40189014 Free PMC article.
-
Regulatory mechanisms of the Hippo/YAP axis by G-protein coupled estrogen receptor in gastric signet-ring cell carcinoma.Neoplasia. 2025 Sep;67:101199. doi: 10.1016/j.neo.2025.101199. Epub 2025 Jun 23. Neoplasia. 2025. PMID: 40554953 Free PMC article.
-
Downregulation of HTRA1 Promotes EMT and Anoikis Resistance in Colorectal Cancer via Activation of Hippo/YAP1 Pathway by Facilitating LATS2 Degradation.Mol Carcinog. 2025 Aug;64(8):1330-1346. doi: 10.1002/mc.23933. Epub 2025 May 26. Mol Carcinog. 2025. PMID: 40418093
-
The role of Hippo/YAP1 in cancer-associated fibroblasts: Literature review and future perspectives.Cancer Lett. 2024 Nov 1;604:217244. doi: 10.1016/j.canlet.2024.217244. Epub 2024 Sep 10. Cancer Lett. 2024. PMID: 39260668 Free PMC article. Review.
-
Exploring Hippo YAP/TAZ Signaling: A Novel Avenue for Cardiovascular Disorders.Cell Biol Int. 2025 Sep;49(9):1079-1101. doi: 10.1002/cbin.70052. Epub 2025 Jul 14. Cell Biol Int. 2025. PMID: 40654305 Review.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48. - PubMed
-
- Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188615. - PubMed
-
- Liu J, Yuan Q, Guo H, Guan H, Hong Z, Shang D. Deciphering drug resistance in gastric cancer: potential mechanisms and future perspectives. Biomed Pharmacother. 2024;173:116310. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

