Unflavored electronic cigarette exposure induces alterations in airway ciliary structure and function
- PMID: 40604837
- PMCID: PMC12224850
- DOI: 10.1186/s12931-025-03302-w
Unflavored electronic cigarette exposure induces alterations in airway ciliary structure and function
Abstract
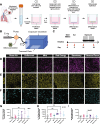
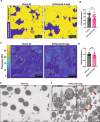
Electronic cigarettes (e-cigs) have been introduced as a safer alternative to traditional combustible cigarettes and have been growing in popularity. E-cig e-liquids all contain the carrier compounds, vegetable glycerin (VG), propylene glycol (PG), and nicotine, together with different flavors, but the effects of inhalation of these compounds on the airway are not well understood. This study investigates the effects of e-cig exposure on primary human airway epithelial cells grown in air-liquid interface (ALI) cultures, specifically focusing on mucociliary clearance, the lung's primary host defense mechanism whereby pathogens and particles trapped by mucus are cleared by unidirectional beating by ciliated cells. We developed a microcontroller-based exposure system to reproducibly examine cellular and molecular changes in ALI cultures from e-cig exposure. Here we show heterogeneous, donor-dependent effects of different e-cig flavors on airway epithelial cells. Examining the effects of the unflavored carrier compounds common to all e-cigs, we found that ALI airway cultures exposed to PG:VG (30:70 ratio) with 5% nicotine unflavored e-cigs show a reduction in ciliary beat frequency. Moreover, using transmission electron microscopy, we identified defects in ciliary ultrastructure induced by unflavored e-cigs. Phosphoproteomic analysis uncovered changes in phosphorylation of proteins involved in cadherin and actin binding and the Rho GTPase signaling pathway, which are all involved in cytoskeletal remodeling that may influence ciliary structure and function. Altogether, our findings suggest that exposure to all e-cigs reduces mucociliary clearance.
Keywords: Ciliary beating frequency; Cytoskeletal remodeling; E-cigarettes; Motile cilia; Phosphoproteomics; Rho GTPase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the UCLA Institutional Review Board (IRB) in accordance with the Declaration of Helsinki. Deidentified human lungs were procured under IRB exemption# 21–000390. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179276/. - PubMed
-
- Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

