SHMT2 overexpression improves glaucoma by enhancing mitophagy in retinal ganglion cells through promoting the phospho of PINK1
- PMID: 40604870
- PMCID: PMC12220441
- DOI: 10.1186/s13000-025-01675-6
SHMT2 overexpression improves glaucoma by enhancing mitophagy in retinal ganglion cells through promoting the phospho of PINK1
Abstract
Background: Glaucoma is a major eye disease that causes blindness. The loss of retinal ganglion cells (RGCs) due to mitophagy impairment is a key driver of glaucoma. SHMT2 depletion leads to an increase in reactive oxygen species (ROS), but its role in regulating mitophagy remains unclear. This study aims to investigate the mechanism by which SHMT2 contributes to glaucoma through the regulation of RGC mitophagy.
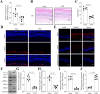
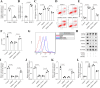
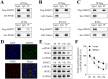
Methods: The role of SHMT2 in glaucoma was evaluated through hematoxylin and eosin (H&E) staining and immunofluorescence (IF) staining of acute ocular hypertension (AOH) mouse eyeballs. Mitophagy was assessed by measuring LDH release, apoptosis, mitochondrial membrane potential, lipid ROS, and the protein levels of mitophagy-related proteins in RGCs. The underlying mechanism was investigated using co-immunoprecipitation, IF staining, and Western blot analysis.
Results: Results showed that SHMT2 expression was decreased in the AOH mouse model. NMDA inhibited mitophagy in RGCs, which was restored by SHMT2 overexpression. Moreover, SHMT2 overexpression stabilized PINK1 expression by enhancing the phosphorylation of PINK1. In vivo experiments suggested that SHMT2 overexpression increased the thickness of the retinal ganglion cell-inner plexiform layer.
Conclusion: This study confirmed that SHMT2 overexpression alleviated glaucoma by enhancing mitophagy in RGCs through the upregulation of PINK1 phosphorylation, suggesting that SHMT2 may serve as a potential therapeutic target for glaucoma.
Keywords: Glaucoma; Mitophagy; PINK1; Retinal ganglion cells; SHMT2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Shanghai Xinshijie Dongqu Eye Hospital. This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. All animal experiments should comply with the ARRIVE guidelines. All methods were carried out in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Dimethyl α-ketoglutarate ameliorates cisplatin-induced acute kidney injury by modulating mitophagy through the PINK1/Parkin pathway.Eur J Med Res. 2025 Aug 13;30(1):746. doi: 10.1186/s40001-025-03010-7. Eur J Med Res. 2025. PMID: 40796901 Free PMC article.
-
A Reduction in Mitophagy Is Associated with Glaucomatous Neurodegeneration in Rodent Models of Glaucoma.Int J Mol Sci. 2024 Dec 4;25(23):13040. doi: 10.3390/ijms252313040. Int J Mol Sci. 2024. PMID: 39684751 Free PMC article.
-
RTA408 alleviates retinal ganglion cells damage in mouse glaucoma by inhibiting excessive autophagy.PLoS One. 2024 Nov 11;19(11):e0313446. doi: 10.1371/journal.pone.0313446. eCollection 2024. PLoS One. 2024. Retraction in: PLoS One. 2025 Jul 14;20(7):e0327995. doi: 10.1371/journal.pone.0327995. PMID: 39527591 Free PMC article. Retracted.
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
Cordycepin Ameliorates Kainic Acid-Induced HT22 Cell Neurotoxicity by Activating GPR120-Mediated Mitophagy.Dev Neurobiol. 2025 Apr;85(2):e22961. doi: 10.1002/dneu.22961. Dev Neurobiol. 2025. PMID: 40007070 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

