Cis,cis-muconic acid production from lignin related molecules byAcinetobacter baylyi ADP1
- PMID: 40604879
- PMCID: PMC12220188
- DOI: 10.1186/s12934-025-02780-3
Cis,cis-muconic acid production from lignin related molecules byAcinetobacter baylyi ADP1
Abstract
Background: Cis,cis-muconic acid (ccMA), an important platform chemical, can be produced from lignin related molecules (LRM) via a specific two-branch catabolic route known as the β-ketoadipate pathway, which is present in certain soil bacteria. This pathway enables high production yields because ccMA is a native intermediate in one of its branches. However, commonly obtained LRM, such as p-coumaric and ferulic acid, are typically metabolized through the branch that lacks the ccMA intermediate. To redirect these LRM toward ccMA production, the two branches must be functionally integrated. This is usually achieved by introducing a non-native enzymatic activity, specifically protocatechuate decarboxylase (PCADC), which catalyzes the conversion of protocatechuate to catechol. Nevertheless, this conversion often represents the rate-limiting step in the production process.
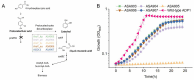
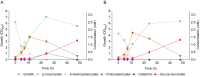
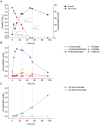
Results: Here, we established a growth-coupled selection system for screening PCADCs using the soil bacterium Acinetobacter baylyi ADP1 as the host. In this system, cell growth depends on the in vivo performance of PCADC, thereby enabling the selection of the optimal candidate for further ccMA production. In total, five PCADC candidates were screened. AGDC1, a gallic acid decarboxylase from the yeast Blastobotrys adeninivorans, was selected for production studies. In fed-batch cultivations, the engineered strain expressing AGDC1 achieved an 83% molar yield of ccMA from ferulate and p-coumarate, that were found in lignin hydrolysate derived from straw.
Conclusion: In this study, we established a growth-based selection system for PCADCs. The outcome of the selection system was further validated in production cultivations using an engineered A. baylyi ADP1 strain. This study not only confirms the feasibility of AGDC1 in ccMA production in bacterial systems but also provides a practical screening system for future improvements.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12934-025-02780-3.
Keywords: Acinetobacter baylyi ADP1; cis; cis-muconic acid; Gallic acid decarboxylase; Growth-coupled selection; Lignin related molecules; Protocatechuate decarboxylase.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
A consortium-based approach to adaptive laboratory evolution of Acinetobacter baylyi ADP1 reveals novel genetic targets for lignin valorization.J Appl Microbiol. 2025 Jun 2;136(6):lxaf138. doi: 10.1093/jambio/lxaf138. J Appl Microbiol. 2025. PMID: 40478763
-
Characterization of Highly Ferulate-Tolerant Acinetobacter baylyi ADP1 Isolates by a Rapid Reverse Engineering Method.Appl Environ Microbiol. 2022 Jan 25;88(2):e0178021. doi: 10.1128/AEM.01780-21. Epub 2021 Nov 17. Appl Environ Microbiol. 2022. PMID: 34788063 Free PMC article.
-
Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity.Metab Eng Commun. 2016 Apr 22;3:111-119. doi: 10.1016/j.meteno.2016.04.002. eCollection 2016 Dec. Metab Eng Commun. 2016. PMID: 29468118 Free PMC article.
-
Acinetobacter baylyi ADP1-naturally competent for synthetic biology.Essays Biochem. 2021 Jul 26;65(2):309-318. doi: 10.1042/EBC20200136. Essays Biochem. 2021. PMID: 33769448 Review.
-
Acinetobacter baylyi ADP1: transforming the choice of model organism.IUBMB Life. 2011 Dec;63(12):1075-80. doi: 10.1002/iub.530. Epub 2011 Oct 27. IUBMB Life. 2011. PMID: 22034222 Review.
References
-
- Shanks BH, Keeling PL. Bioprivileged molecules: creating value from biomass. Green Chem. 2017;19:3177–85.
-
- Polen T, Spelberg M, Bott M. Toward biotechnological production of adipic acid and precursors from biorenewables. J Biotechnol. 2013;167:75–84. - PubMed
-
- Xie N-Z, Liang H, Huang R-B, Xu P. Biotechnological production of muconic acid: current status and future prospects. Biotechnol Adv. 2014;32:615–22. - PubMed
-
- Lu R, Lu F, Chen J, Yu W, Huang Q, Zhang J, et al. Production of diethyl terephthalate from Biomass-Derived muconic acid. Angew Chem Int Ed. 2016;55:249–53. - PubMed
LinkOut - more resources
Full Text Sources

