LPS pretreated dental follicle stem cell derived exosomes promote periodontal tissue regeneration via miR-184 and PPARα-Akt-JNK signaling pathway
- PMID: 40605003
- PMCID: PMC12224499
- DOI: 10.1186/s13287-025-04462-8
LPS pretreated dental follicle stem cell derived exosomes promote periodontal tissue regeneration via miR-184 and PPARα-Akt-JNK signaling pathway
Abstract
Purpose: Lipopolysaccharide (LPS) pretreated dental follicle stem cells (DFSCs)-derived exosomes (L-D-Exo) exhibit enhanced therapeutic effects in periodontitis treatment, but the effective components responsible for these effects remain unidentified. The aim of this study is to investigate the differences in expression profile and regulatory effect of the exosomal microRNAs (miRNAs) from DFSCs and PDLSCs on periodontal tissue regeneration.
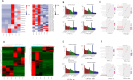
Methods: High-throughput miRNA sequencing was performed on DFSCs and PDLSCs derived exosomes under both Porphyromonas gingivalis (P.g) LPS pretreatment and normal conditions. Through bioinformatic analysis, miR-184 was selected as the key miRNA due to its specific down-regulation in L-D-Exo, which linked to oxidative stress regulation. After changing the expression of miR-184 in PDLSCs, the fluorescence intensity of reactive oxygen species (ROS), malondialdehyde (MDA) content and antioxidant related enzyme activities, and the expression levels of inflammatory cytokines and osteogenesis-related genes in PDLSCs were detected. In addition, dual-luciferase reporter assay and Western blot were used to explore the target gene and downstream signaling pathways. In vivo, miR-184 Antagomir was injected into mice with experimental periodontitis to evaluate the role and mechanism of miR-184 in periodontal tissue regeneration.
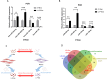
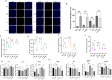
Results: Inhibition of miR-184 in PDLSCs significantly impaired oxidative stress, as evidenced by decreased ROS fluorescence intensity and MDA content, alongside increased activities of antioxidant enzymes. This reduction in oxidative stress subsequently decreased the expression of intracellular inflammatory cytokines, while promoting the expression of osteogenic genes. The dual-luciferase reporter assay confirmed the direct binding of miR-184 with Peroxisome proliferator-activated receptor α (PPARα). MiR-184 inhibition activated the downstream protein kinase B (Akt) pathway and inhibited the c-Jun N-terminal kinase (JNK) pathway under inflammatory conditions. Furthermore, miR-184 Antagomir application also enhanced the therapeutic efficacy of periodontitis mice by reducing inflammation and promoting periodontal osteogenesis.
Conclusion: Inhibition of miR-184 facilitates periodontal regeneration, which targets the PPARα-Akt-JNK signaling pathway to suppress oxidative stress in periodontal tissues.
Keywords: Dental follicle stem cells; Exosomes; MicroRNA; Oxidative stress; Periodontal tissue regeneration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent was obtained from all patients under ethical approval (WCHSIRB-D-2023-165, titled “Dental follicle stem cell-derived exosomes promote periodontal tissue regeneration via miR-184 and its mechanisms”) by the Ethics committee of West China Hospital of Stomatology (date of approval: November 16, 2023) before utilizing donated dental specimens for further study. The animal study was performed according to the Institutional Animal Care and Use Committee (IACUC) at Sichuan University, and approved (WCHSIRB-D-2023-037, titled “Dental follicle stem cell-derived exosomes promote periodontal tissue regeneration via miR-184 and its mechanisms”) by the Ethics Committee of West China Hospital of Stomatology (date of approval: February 16, 2023). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis—a comprehensive review. J Clin Periodontol. 2017;44(Suppl 18):S94-s105. - PubMed
-
- Slots J. Periodontitis: facts, fallacies and the future. Periodontology 2000. 2017;75(1):7–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

