DSG3 promotes bladder cancer growth and metastasis via AKT/GSK3β/β-catenin pathway
- PMID: 40605005
- PMCID: PMC12225158
- DOI: 10.1186/s12967-025-06754-2
DSG3 promotes bladder cancer growth and metastasis via AKT/GSK3β/β-catenin pathway
Abstract
Background: The high metastasis rate is the primary contributor to the high mortality rate associated with muscle-invasive bladder cancer (MIBC). Therefore, elucidating the mechanisms involved and identifying potential therapeutic targets are crucial for improving the overall prognosis of bladder cancer (BLCA).
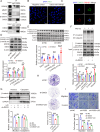
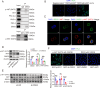
Methods: We used consensus clustering and differential gene expression analyses to identify the key gene desmoglein 3 (DSG3). Subsequently, we examined the expression of DSG3 in BLCA and its association with the clinical characteristics and prognosis. Comprehensive in vitro and in vivo experiments were conducted to elucidate the functions of DSG3 and the reasons behind the upregulation of DSG3 in BLCA, as well as to investigate the mechanisms by which DSG3 promotes metastasis.
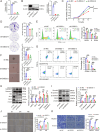
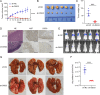
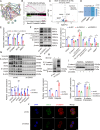
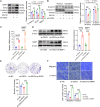
Results: DSG3 was markedly upregulated in BLCA, particularly in the basal/squamous (Ba/Sq) subtype. Importantly, elevated DSG3 levels demonstrated a strong association with aggressive tumor behavior and poorer clinical outcomes. Functional experiments revealed that DSG3 knockdown significantly impeded cancer stemness characteristics, epithelial-mesenchymal transition (EMT), migration, and invasion capabilities in vitro, whereas in vivo studies showed marked reductions in tumorigenesis and lung metastasis. Mechanistic investigations indicated that STAT3 transcriptionally activated DSG3 expression in BLCA cells. Downstream pathway analysis further showed that DSG3 promoted AKT phosphorylation, thereby inhibiting GSK3β activity. This molecular pathway promoted β-catenin nuclear translocation, thereby triggering transcriptional upregulation of SOX2 and MMP7 expression, ultimately mediating BLCA progression.
Conclusion: Our study demonstrates a novel mechanism by which DSG3 enhances cancer stemness, EMT, migration, and invasive capabilities through upregulation of SOX2 and MMP7 expression through the AKT/GSK3β/β-catenin pathway, ultimately leading to growth and metastasis of BLCA. This study elucidated the role of DSG3 in BLCA and its mechanism in activating the Wnt/β-catenin signaling pathway. We anticipate this study will identify potential biomarkers for predicting progression and for assessing prognosis. Furthermore, this study introduced a novel intervention target for BLCA treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University, China, (approval number: 2023-KY-1216-004). The animal study protocol was approved by the Life Science Ethics Review Committee of Zhengzhou University, (approval number: ZZUIRB2022-143). Patient’s specimen and clinical case information were collected following the declaration of Helsinki and Istanbul. Written informed consent was obtained from all the participants or legal guardians. The study was conducted with archived specimens. Consent for publication: All patient data were fully anonymized prior to analysis, and no individual identifiers were retained. Therefore, consent for publication was not required as per institutional guidelines and international ethical standards. Competing interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
SFXN1 promotes bladder cancer metastasis by restraining PINK1-dependent mitophagy.Oncogene. 2025 Aug;44(32):2893-2906. doi: 10.1038/s41388-025-03460-7. Epub 2025 Jun 4. Oncogene. 2025. PMID: 40468051 Free PMC article.
-
CHD4 drives gastric cancer metastasis via MYH9/GSK3β/β-catenin axis and WNT/EMT pathway activation.Cancer Lett. 2025 Sep 28;628:217813. doi: 10.1016/j.canlet.2025.217813. Epub 2025 Jun 4. Cancer Lett. 2025. PMID: 40480417
-
Caveolin-1 inhibits the proliferation and invasion of lung adenocarcinoma via EGFR degradation.Sci Rep. 2025 Jul 1;15(1):21654. doi: 10.1038/s41598-025-05259-8. Sci Rep. 2025. PMID: 40594106 Free PMC article.
-
Role of Mesenchymal Markers in Colorectal Cancer Metastasis.Mol Biol Rep. 2025 Jul 4;52(1):673. doi: 10.1007/s11033-025-10745-3. Mol Biol Rep. 2025. PMID: 40613936 Review.
-
A review: hippo signaling pathway promotes tumor invasion and metastasis by regulating target gene expression.J Cancer Res Clin Oncol. 2021 Jun;147(6):1569-1585. doi: 10.1007/s00432-021-03604-8. Epub 2021 Apr 17. J Cancer Res Clin Oncol. 2021. PMID: 33864521 Free PMC article. Review.
References
-
- Filho AM, Laversanne M, Ferlay J, Colombet M, Pineros M, Znaor A, Parkin DM, Soerjomataram I, Bray F. The Globocan 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2024;11. 10.1002/ijc.35278. - PubMed
-
- Lopez-Beltran A, Cookson MS, Guercio BJ, Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ. 2024;384:22. 10.1136/bmj-2023-076743. - PubMed
-
- Samnani S, Veitch ZWN, Kaiser J, Stecca C, Alqaisi H, North SA, Sridhar SS, Alimohamed NS. Trends over time in survival in patients with urothelial carcinoma in the real-world: a multicenter analysis. J Clin Oncol. 2021;39:2. 10.1200/JCO.2021.39.6_suppl.412.
-
- Warrick JI, Sjodahl G, Kaag M, Raman JD, Merrill S, Shuman L, Chen G, Walter V, DeGraff DJ. Intratumoral heterogeneity of bladder cancer by molecular subtypes and histologic variants. Eur Urol. 2019;75:18–22. 10.1016/j.eururo.2018.09.003. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

