Multicenter study correlating molecular characteristics and clinical outcomes of cancer cases with patient-derived organoids
- PMID: 40605011
- PMCID: PMC12220348
- DOI: 10.1186/s13046-025-03437-0
Multicenter study correlating molecular characteristics and clinical outcomes of cancer cases with patient-derived organoids
Abstract
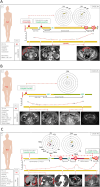
Background: 3D-spatial interaction between cancer cells influences tumor behavior, making it essential to replicate tumor structures for predicting patient outcomes.
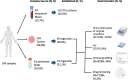
Methods: We collected data from three multicenter prospective studies to evaluate the ability to establish Patient-Derived Organoids (PDOs) from different biological samples and timepoints, correlating their characteristics and drug sensitivity with clinical outcomes.
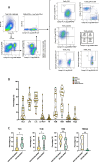
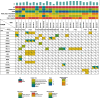
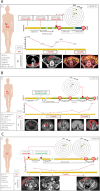
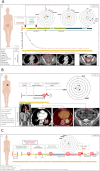
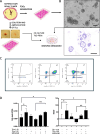
Results: From 184 patients (17 tumor types), 249 samples were collected: 149 (59.8%) from tumor tissue, 61 (24.5%) from peritoneal fluids, 39 (15.7%) from peripheral blood. Success rates for PDO establishment were 39.5%, 34.4%, and 25.6%, respectively. PDOs reproduced pathological and immunohistochemical patterns of source tumors, with pathogenic variants confirmed in 84% (21/25). In a series of 13 baseline and sequential PDOs from 9 patients undergoing treatment, responses to therapy mirrored patient responses during therapy.
Conclusions: PDOs preserve tumor features, reflect disease progression, and predict treatment responses, providing valuable models to complement molecular testing in precision medicine.
Keywords: Drug screening; Patient-derived organoids; Precision Medicine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures performed in human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided informed consent prior to their participation. Name of the ethics committee that approved the study: Comité HM Hospitales and the committee’s reference number: 18.09.1289.GHM. Consent for publication: All participants provided informed consent prior to their participation, including consent for publication. Competing interests: Jesus Garcia-Donas: Honoraria: Pfizer, BMS, Roche, MSD, Astra Zenenca, Ipsen, Astellas, Merck, Takeda, Healthincode, Novartis; Research grants: Pfizer, BMS, Roche, MSD, Astra Zenenca, Ipsen, Astellas, Merck, Takeda, Healthincode, Novartis; Travel expenses: Pfizer, BMS, Roche, MSD, Astra Zenenca, Ipsen, Astellas, Merck, Takeda, Healthincode, Novartis.
Figures


References
-
- https://gco.iarc.fr/today/home. Available from: https://gco.iarc.fr/today/home.
-
- Rodon J, Soria JC, Berger R, Miller WH, Rubin E, Kugel A, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25(5):751–8. Available from: https://www.nature.com/articles/s41591-019-0424-4. - PMC - PubMed
-
- Navarro P, Beato C, Rodriguez-Moreno JF, Ruiz-Llorente S, Mielgo X, Pineda E, et al. Prospective study of the real impact of fusion centered genomic assays in patient management in a national collaborative group: the GETHI-XX-16 study. Clin Transl Oncol. 2024;27(6):2719–30. 10.1007/s12094-024-03745-5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

