Ultraviolet B-induced oxidative damage in human skin keratinocytes is alleviated by Pinus morrisonicola leaf essential oil through activation of the Nrf2-dependent antioxidant defense system
- PMID: 40605175
- PMCID: PMC12231284
- DOI: 10.1080/13510002.2025.2527427
Ultraviolet B-induced oxidative damage in human skin keratinocytes is alleviated by Pinus morrisonicola leaf essential oil through activation of the Nrf2-dependent antioxidant defense system
Abstract
Background: Ultraviolet B (UVB) radiation contributes to skin disorders such as photodamage, photoaging, and cancer. Natural antioxidants can mitigate UVB-induced damage. Pinus morrisonicola (Taiwan white pine), known for its anti-cancer, anti-inflammatory, and antioxidant properties, is used in health-promoting beverages, but its skin-protective effects remain underexplored.
Purpose: This study investigates the protective effects of P. morrisonicola leaf essential oil (PMLEO) against UVB-induced damage in HaCaT keratinocytes.
Methods: HaCaT cells were exposed to UVB and treated with PMLEO. Cell viability, reactive oxygen species (ROS) levels, and antioxidant enzyme expression were assessed. The role of Nrf2, a key antioxidant regulator, was evaluated through knockdown experiments. The effects on UVB-induced melanogenesis were examined via α-MSH secretion followed by p53-mediated POMC expression.
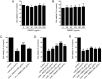
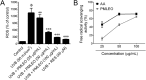
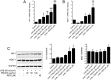
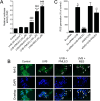
Results: PMLEO and P. morrisonicola bark essential oil (PMBEO) were non-cytotoxic up to 200 µg/mL. UVB reduced cell viability to 43%, but PMLEO co-treatment significantly restored viability and reduced ROS levels via Nrf2 activation, increasing NQO-1 and HO-1. Nrf2 knockdown impaired PMLEO's protection. PMLEO also inhibited UVB-induced α-MSH secretion by downregulating p53-mediated POMC expression, suggesting an anti-melanogenic effect.
Conclusion: PMLEO protects dermal keratinocytes against UVB-induced oxidative stress, cell death, and melanogenesis via Nrf2 activation, highlighting its potential as a natural skin protectant.
Keywords: HaCaT; Nrf2 pathway; Pinus morrisonicola; antioxidant; essential oil; photoaging; photodamage; ultraviolet B.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Gilchrest BA . Photoaging. J Invest Dermatol. 2013;133(E1):E2–E6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
