A proof-of-concept study of pitolisant for excessive daytime sleepiness in patients with Prader-Willi syndrome
- PMID: 40605372
- PMCID: PMC12582198
- DOI: 10.5664/jcsm.11800
A proof-of-concept study of pitolisant for excessive daytime sleepiness in patients with Prader-Willi syndrome
Abstract
Study objectives: The majority of patients with Prader-Willi syndrome experience excessive daytime sleepiness (EDS). This study evaluated the effects of pitolisant, a histamine 3 (H3)-receptor antagonist/inverse agonist that promotes wakefulness, in patients with Prader-Willi syndrome and EDS.
Methods: In this phase 2, randomized, double-blind, placebo-controlled, proof-of-concept study, patients ages 6-65 years with a confirmed diagnosis of Prader-Willi syndrome with EDS were randomized 1:1:1 to receive lower-dose pitolisant (children/adolescents/adults, 8.9/13.35/17.8 mg), higher-dose pitolisant (children/adolescents/adults, 17.8/26.7/35.6 mg), or matching placebo for 11 weeks (3-week titration/8-week maintenance). The primary endpoint was change from baseline to week 11 in Epworth Sleepiness Scale for Children and Adolescents (parent/caregiver version) score. Other measures included the Caregiver Global Impression of Severity for EDS, Aberrant Behavior Checklist-Community, second edition, and Hyperphagia Questionnaire for Clinical Trials.
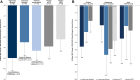
Results: Of 65 patients randomized and treated, 59 (90.8%) completed the double-blind phase. Least-squares (LS) mean improvement from baseline to week 11 in Epworth Sleepiness Scale for Children and Adolescents score was greater for higher-dose pitolisant (-5.0) vs placebo (-3.9; LS mean [standard error] difference, -1.1 [1.52]), but not for lower-dose pitolisant (-3.5) vs placebo (LS mean [standard error] difference, 0.5 [1.6]). The largest effect of pitolisant was seen in children (ages 6 to < 12 years; LS mean [standard error] difference for higher-dose pitolisant vs placebo, -3.5 [1.90]). Improvements were observed across other measures, especially in the higher-dose pitolisant group, including LS mean (standard error) change of -5.5 (1.2) on the irritability domain of the Aberrant Behavior Checklist-Community, second edition, and -3.1 (1.0) on the Hyperphagia Questionnaire for Clinical Trials. The most common adverse events in pitolisant-treated patients (doses pooled) were anxiety, irritability, and headache (11.9% each), consistent with the known safety profile of pitolisant.
Conclusions: Results of this proof-of-concept study support further evaluation of pitolisant in patients with Prader-Willi syndrome and EDS.
Clinical trial registration: Registry: ClinicalTrials.gov; Name: A Phase 2 Study to Evaluate the Safety and Efficacy of Pitolisant in Patients With Prader-Willi Syndrome, Followed by an Open Label Extension; URL: https://clinicaltrials.gov/study/NCT04257929; Identifier: NCT04257929.
Citation: Revana A, Bhattacharjee R, Miller JL, et al. A proof-of-concept study of pitolisant for excessive daytime sleepiness in patients with Prader-Willi syndrome. J Clin Sleep Med. 2025;21(11):1893-1902.
Keywords: H3 histamine receptor antagonists; behavioral symptoms; excessive daytime sleepiness; histamine H3 receptor; proof-of-concept study.
© 2025 The Authors.
Conflict of interest statement
All authors have seen and approved the manuscript. A.R. reports serving as an investigator for Harmony Biosciences and a consultant to TREND, LLC. R.B. reports serving as a consultant to Avadel Pharmaceuticals, Harmony Biosciences, Jazz Pharmaceuticals, and the Institute for Advanced Clinical Trials; and receiving speaker fees for Harmony Biosciences. J.L.M. reports receiving research funding from Harmony Biosciences, Rhythm Pharmaceuticals, Soleno Therapeutics, and TRYP Therapeutics. A.C. reports serving as a consultant to BioMarin Pharmaceuticals. P.K. and S.R. report nothing to disclose. G.R., E.B., K.D.R., D.S., K.B., and J.D. are employees of Harmony Biosciences, Plymouth Meeting, PA. Editorial and medical writing assistance was provided under the direction of the authors by Adrienne Drinkwater, PhD, and Pratibha Hebbar, PhD, Synchrony Medical Communications, LLC, West Chester, PA, and funded by Harmony Biosciences, LLC, Plymouth Meeting, PA.
Figures


References
-
- Cassidy SB , Schwartz S , Miller JL , Driscoll DJ . Prader-Willi syndrome . Genet Med. 2012. ; 14 ( 1 ): 10 – 26 . - PubMed
-
- Tauber M , Hoybye C . Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction . Lancet Diabetes Endocrinol. 2021. ; 9 ( 4 ): 235 – 246 . - PubMed
-
- Camfferman D , McEvoy RD , O’Donoghue F , Lushington K . Prader Willi syndrome and excessive daytime sleepiness . Sleep Med Rev. 2008. ; 12 ( 1 ): 65 – 75 . - PubMed
-
- Ghergan A , Coupaye M , Leu-Semenescu S , et al . Prevalence and phenotype of sleep disorders in 60 adults with Prader-Willi syndrome . Sleep. 2017. ; 40 ( 12 ): zsx162 .
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

