Breast implant illness after reconstruction with silicone breast implants
- PMID: 40605595
- PMCID: PMC12342748
- DOI: 10.1093/jnci/djaf136
Breast implant illness after reconstruction with silicone breast implants
Abstract
Background: "Breast implant illness" (BII) is a constellation of non-specific constitutional, rheumatologic, mental, and cognitive symptoms reported increasingly by women carrying silicone breast implants (SBIs). The impact of BII on the well-being of breast cancer patients with SBI-based breast reconstructions is a subject of debate.
Methods: In a multicenter cohort of breast cancer survivors (n = 9590) treated between 2000 and 2015 in 6 major regional hospitals in the Netherlands, we performed a health survey (response rate 64.7%). The presence of 18 BII-associated symptoms was compared between patients with and without SBIs in multivariable logistic regression models. In a latent class analysis (LCA), distinct symptom patterns were identified in the study population.
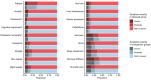
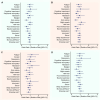
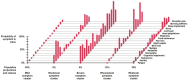
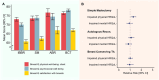
Results: Median follow-up time was 13.7 (IQR, 6.8) years. Of all SBI-exposed patients (n = 1821), 20.7% reported ≥4 BII-associated symptoms vs 21.2% of non-exposed patients (risk ratio 0.98; 95% CI = 0.88 to 1.09). Joint pain, sicca, sleep impairment, morning stiffness, and shoulder pain were reported most frequently. Patients with SBIs did not have a significantly increased risk of any of the individual BII-associated symptoms. The LCA identified 5 distinct symptom clusters. Patients with SBI-exposure had a lower risk of falling in the most severe symptom cluster (odds ratio 0.64; 95% CI = 0.43 to 0.96). The other symptom clusters were not significantly associated with SBI-exposure.
Conclusions: Our results indicate that breast cancer survivors with SBI-based reconstructions do not experience more BII-associated symptoms than breast cancer survivors without SBIs, challenging the notion of BII as a distinct clinical entity based on a generic silicone-induced biomechanical pathophysiological mechanism.
Trial registration: This study was preregistered at ClinicalTrials.gov on June 2, 2022 (NCT05400954).
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors confirm that there are no financial or non-financial competing interests to report.
Figures
Comment in
-
Breast implant illness and the power of national mandatory reporting.J Natl Cancer Inst. 2025 Aug 1;117(8):1536-1538. doi: 10.1093/jnci/djaf156. J Natl Cancer Inst. 2025. PMID: 40673928 No abstract available.
References
-
- Lim DW, Metcalfe KA, Narod SA. Bilateral mastectomy in women with unilateral breast cancer: a review. JAMA Surg. 2021;156:569-576. - PubMed
-
- Kouwenberg CAE, de Ligt KM, Kranenburg LW, et al. Long-term health-related quality of life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast Reconstr Surg. 2020;146:1-13. - PubMed
-
- Fryzek JP, Signorello LB, Hakelius L, et al. Self-reported symptoms among women after cosmetic breast implant and breast reduction surgery. Plast Reconstr Surg. 2001;107:206-213. - PubMed
-
- Taskindoust M, Bowman T, Thomas SM, et al. The patient narrative for breast implant illness: a 10-year review of the U.S. Food and Drug Administration’s MAUDE Database. Plast Reconstr Surg. 2022;150:1181-1187. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

