NEDD4 Promotes Sertoli Cell Proliferation and Adult Leydig Cell Differentiation in the Murine Testis
- PMID: 40605617
- PMCID: PMC12280327
- DOI: 10.1210/endocr/bqaf115
NEDD4 Promotes Sertoli Cell Proliferation and Adult Leydig Cell Differentiation in the Murine Testis
Abstract
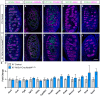
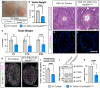
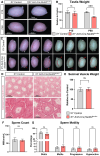
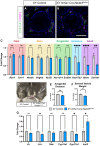
Successful testis development relies on the coordinated differentiation and assembly of various cell types to establish both endocrine and reproductive functions. The ubiquitin ligase NEDD4 has emerged as a key player in murine testis development, with this enzyme being implicated in gonadal sex determination and spermatogonial stem cell differentiation. Here, we report hitherto uncharacterized roles of NEDD4 in postnatal testis development. Utilizing Nr5a1- and Amh-Cre drivers to conditionally ablate Nedd4 in testicular somatic cells, we show that NEDD4 promotes Sertoli cell proliferation through the modulation of the PI3K-AKT signaling pathway. This ubiquitin ligase also ensures proper differentiation of adult Leydig cells and may contribute to murine steroidogenesis. Furthermore, NEDD4 is essential for adrenal gland differentiation, as its loss results in adrenal dysgenesis. These findings highlight NEDD4 as a crucial factor in testis development, emphasizing the importance of ubiquitination and post-translational modifications in reproductive biology.
Keywords: NEDD4; adrenal dysgenesis; steroidogenesis; testis development; testis function.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Windley SP, Wilhelm D. Signaling pathways involved in mammalian sex determination and gonad development. Sex Dev. 2015;9(6):297‐315. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

