Lamin A/C Expression in Hematopoietic Cells Declines During Human Aging and Constrains Atherosclerosis in Mice
- PMID: 40605747
- PMCID: PMC12379799
- DOI: 10.1161/ATVBAHA.124.322893
Lamin A/C Expression in Hematopoietic Cells Declines During Human Aging and Constrains Atherosclerosis in Mice
Abstract
Background: Aging is the primary risk factor for atherosclerosis, a degenerative process regulated by immune cells and the leading cause of death worldwide. Previous studies on premature aging syndromes have linked atherosclerosis to defects in A-type lamins, key nuclear envelope components. However, whether these defects influence atherosclerosis during normal aging remains unexplored. Here, we examined how aging affects lamin A/C expression in circulating leukocytes and investigated the impact of manipulating their expression in hematopoietic cells on their function and atherosclerosis progression.
Methods: Flow cytometry assessed lamin A/C expression in human circulating leukocytes. Bone marrow from donor mice was transplanted into lethally irradiated, Ldlr-/--deficient mice to study leukocyte extravasation into the vessel wall via intravital microscopy in the cremaster muscle, and high-fat-diet-induced atherosclerosis via Oil Red O staining of the aorta and carotid arteries. Single-cell RNA sequencing of the aorta was conducted to identify transcriptional changes associated with hematopoietic cell lamin A/C gain-of-function or loss-of-function.
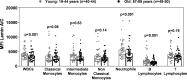
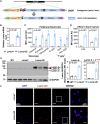
Results: Human aging is associated with lower levels of lamin A/C expression in blood-borne leukocytes. To evaluate the functional relationship between hematopoietic lamin A/C expression and atherosclerosis development, we used Lmna-null mice and Lmnatg mice, the latter being the first in vivo model of lamin A gain-of-function. Transplanting lamin A/C-deficient bone marrow into Ldlr-/- mice increased leukocyte extravasation into the vessel wall and accelerated atherosclerosis. Conversely, transplantation of bone marrow overexpressing lamin A into Ldlr-/- receptor mice reduced leukocyte extravasation and atherosclerosis. Single-cell RNA sequencing of atherosclerotic mouse aorta revealed that alterations to hematopoietic cell lamin A/C expression primarily modify the transcriptome of immune cell populations and endothelial cells, affecting their functionality.
Conclusions: We suggest that the age-related decline in lamin A/C expression in blood-borne immune cells contributes to increased leukocyte extravasation and atherosclerosis, highlighting lamin A/C as a novel regulator of age-related atherosclerosis.
Keywords: aging; atherosclerosis; cardiovascular diseases; inflammation; lamin type A; single-cell gene expression analysis.
Conflict of interest statement
H. Bueno received consulting/speaking fees from AstraZeneca, Novartis, Novo Nordisk, and Organon, unrelated to the current study. The funders had no role in the design of the study, the collection, analysis, or interpretation of the data, or the reporting of the study. The other authors report no conflicts.
Figures





References
-
- Libby P. Fanning the flames: inflammation in cardiovascular diseases. Cardiovasc Res. 2015;107:307–309. doi: 10.1093/cvr/cvv188 - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156 - PubMed
-
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58 - PubMed
-
- Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, Berger JS. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. 2013;61:1736–1743. doi: 10.1016/j.jacc.2013.01.054 - PubMed
-
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

