Patient-Reported Well-Being in Value-Based Routine Care Using Tildrakizumab: 52-week Interim Data of the Phase IV Positive Study
- PMID: 40605963
- PMCID: PMC12219165
- DOI: 10.2147/PTT.S526748
Patient-Reported Well-Being in Value-Based Routine Care Using Tildrakizumab: 52-week Interim Data of the Phase IV Positive Study
Abstract
Purpose: Psoriasis profoundly impairs patients' social, emotional, and physical condition, impacting on their overall well-being. Tildrakizumab is an interleukin-23p19 inhibitor labelled for the treatment of moderate-to-severe plaque psoriasis. The main objective of this study was to assess the effect of tildrakizumab on the overall well-being of people with psoriasis. Effectiveness, quality of life (QoL), symptomatology, treatment satisfaction, and the impact of psoriasis on the patients' partners were also evaluated.
Patients and methods: POSITIVE is a 24-month observational study in adults with moderate-to-severe psoriasis treated with tildrakizumab in a real-world setting (ClinicalTrials.gov ID: NCT04823247). Outcome measurements included the 5-item WHO Well-being Index (WHO-5), Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index-Relevant (DLQI-R), Treatment Satisfaction Questionnaire for Medication (TSQM-9), and FamilyPso. We report 52-week (W52) interim data (N = 400; observed cases).
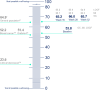
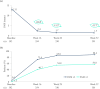
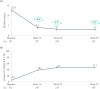
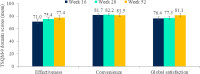
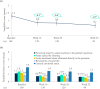
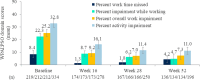
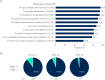
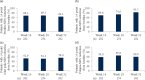
Results: Mean ± 95% CI WHO-5 score increased from 53.8 ± 2.2 at baseline to 66.0 ± 2.3/65.7 ± 2.7 at W28/W52 (p < 0.0001, both). Mean ± 95% CI PASI decreased from 13.1 ± 0.8 at baseline to 1.7 ± 0.3/1.5 ± 0.3 at W28/W52 (p < 0.0001, both). At W28 and W52, 85.8%/54.8% and 88.4%/56.8% of patients achieved PASI ≤ 3/≤ 1. Mean ± 95% CI DLQI-R score decreased from 12.6 ± 0.8 at baseline to 3.3 ± 0.6/3.1 ± 0.6 at W28/W52 (p < 0.0001, both). At W52, mean ± 95% CI TSQM-9 domain scores were 77.4 ± 3.2 for effectiveness, 81.5 ± 2.6 convenience, and 81.1 ± 2.6 global satisfaction. Mean ± 95% CI total FamilyPso decreased from 1.3 ± 0.1 at baseline to 0.7 ± 0.2 at W52 (p < 0.0001). At the point of this analysis, 24.0% of patients had ≥1 adverse event (AE). Only one patient discontinued due to a treatment-related AE.
Conclusion: Tildrakizumab successfully contributes to value-based long-term health care for moderate-to-severe psoriasis by increasing patient wellbeing, QoL and clinical outcomes while showing very good safety and tolerability.
Keywords: RWE; WHO-5 Well-being Index; effectiveness; psoriasis; real-world evidence; tildrakizumab; well-being.
© 2025 Mrowietz et al.
Conflict of interest statement
UM has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall, Amgen, Aristea, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Formycon, Immunic, Janssen-Cilag, LEO Pharma, Merck, Sharp & Dohme, MetrioPharm, Novartis, Phi-Stone, Sanofi-Aventis, UCB Pharma, UNION therapeutics. RS has been an advisor and/or received speaker honoraria and/or received grants from AbbVie, Almirall, Beiersdorf, Janssen, Leo Pharma, Novartis and UCB. SG has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Affibody AB, Akari Therapeutics Plc, Almirall-Hermal, Amgen, Anaptys Bio, Argenx BV, AstraZeneca AB, Biogen Idec, Bioskin, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly, Forward Pharma, Galderma, Hexal AG, Incyte Inc., Janssen-Cilag, Johnson & Johnson, Kymab, Leo Pharma, Medac, MSD, Neubourg Skin Care GmbH, Novartis, Pfizer, Principia Biopharma, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Sanofi-Aventis, Trevi Therapeutics, and UCB Pharma. ZR has been an advisor, investigator, and/or speaker for AbbVie, Alumis, Amgen, Almirall, Avene, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Janssen-Cilag, Leo-Pharma, MEDAC, MSD, Novartis, Pierre Fabre Dermatologie, Pfizer, Sanofi, Takeda and UCB. WW has received speaker and/or consulting honoraria and/or travel refunds from AbbVie, Almirall, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma, Merck Sharp & Dohme, Novartis, Pfizer, and Sandoz. ED has the following conflict of interests: Advisory Board member, consultant, grants, research support, participation in clinical trials, honorarium for speaking, research support, with the following pharmaceutical companies: AbbVie/Abbott, Almirall, Amgen, Boehringer-Ingelheim, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, Lilly, and UCB. J-TM has served as advisor and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, and UCB. P-DG has received speaker and investigator fees or grants from AbbVie, Almirall, Amgen, BMS, Eli Lilly, Flen, Galderma, Janssen, LEO Pharma, Maruho, Meda, Menarini, MSD, Novartis, PellePharm, Pfizer, Serono, UCB and Viatris. PL received honoraria and/or grants as an investigator, speaker, and/or advisory board member for AbbVie, Almirall, Actelion, Celgene, Janssen, Galderma, Lilly, Sanofi, Leo, UCB, Novartis, Pfizer. LN received consultation fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, IBSA, Menarini, Janssen, Novartis, and Sanofi. EdJ has received research grants for the independent research fund of the department of dermatology of the Radboud University Medical Center Nijmegen, The Netherlands from AbbVie, Leo Pharma, Janssen Pharmaceuticals, Novartis, Pfizer, and UCB and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Almirall, Celgene, Galapagos, Janssen Pharmaceutica, Leo Pharma, Lilly, Novartis, Sanofi, and UCB. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands. SM is employed as a scientific officer at the International Federation of Psoriasis Associations (IFPA), receiving no individual compensation. In this role, IFPA has engaged in consultancy services for pharmaceutical companies, including Boehringer Ingelheim, Novartis, Almirall, and UCB. Over the past 12 months, IFPA has received grants and funding from AbbVie, Almirall, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, UCB, and Takeda. VK, EM, AD, KGdJ, and IK are employees of Almirall. MA has received consulting fees and/or speaker honoraries and/or institutional research support from the following pharmaceutical companies manufacturing drugs for psoriasis: AbbVie, Almirall, Amgen, Biogen, Boehringer, Bristol Myers Squibb, Celgene, Celltrion, Centocor, Eli Lilly, Fresenius, GSK, Hexal, Janssen, Klinge, LEO, MC2, Medac, Merck, MSD, Novartis, Pfizer, Sandoz, Sun, UCB and Viatris. The authors report no other conflicts of interest in this work.
Figures

References
-
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–284. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

