Toward a role for the acoustic field in cells interaction
- PMID: 40606011
- PMCID: PMC12213812
- DOI: 10.3389/fnsys.2025.1484769
Toward a role for the acoustic field in cells interaction
Abstract
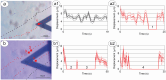
Nanoscale motility of cells is a fundamental phenomenon, closely associated with biological status and response to environmental solicitations, whose investigation has disclosed new perspectives for the comprehension of cell behavior and fate. To investigate intracellular interactions, we designed an experiment to monitor movements of clusters of neuroblastoma cells (SH-SY5Y) growing on a nanomechanical oscillator (nanomotion sensor) suspended few hundreds of microns over the surface of a Petri dish where other neuroblastoma cells are freely moving. We observed that the free-to-move cells feel the presence of cells on the nearby nanosensor (at a distance of up to 300 microns) and migrate toward them, even in presence of environmental hampering factors, such as medium microflows. The interaction is bidirectional since, as evidenced by nanomotion sensing, the cells on the sensor enhance their motion when clusters of freely moving cells approach. Considering the geometry and environmental context, our observations extend beyond what can be explained by sensing of chemical trackers, suggesting the presence of other physical mechanisms. We hypothesize that the acoustic field generated by cell vibrations can have a role in the initial recognition between distant clusters. Integrating our findings with a suitable wave propagation model, we show that mechanical waves produced by cellular activity have sufficient energy to trigger mechanotransduction in target cells hundreds of microns away. This interaction can explain the observed distance-dependent patterns of cellular migration and motion alteration. Our results suggest that acoustic fields generated by cells can mediate cell-cell interaction and contribute to signaling and communication.
Keywords: acoustic field; cell behavior; cell-cell interactions; mechanical waves; nanomotion sensor; neuroblastoma cell.
Copyright © 2025 Girasole, Moretti, Di Giannatale, Di Paolo, Galardi, Lampis, Dinarelli and Longo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

