Non-contrast computed tomography radiomics model to predict benign and malignant thyroid nodules with lobe segmentation: A dual-center study
- PMID: 40606044
- PMCID: PMC12210200
- DOI: 10.4329/wjr.v17.i6.106682
Non-contrast computed tomography radiomics model to predict benign and malignant thyroid nodules with lobe segmentation: A dual-center study
Abstract
Background: Accurate preoperative differentiation of benign and malignant thyroid nodules is critical for optimal patient management. However, conventional imaging modalities present inherent diagnostic limitations.
Aim: To develop a non-contrast computed tomography-based machine learning model integrating radiomics and clinical features for preoperative thyroid nodule classification.
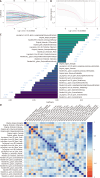
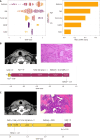
Methods: This multicenter retrospective study enrolled 272 patients with thyroid nodules (376 thyroid lobes) from center A (May 2021-April 2024), using histopathological findings as the reference standard. The dataset was stratified into a training cohort (264 lobes) and an internal validation cohort (112 lobes). Additional prospective temporal (97 lobes, May-August 2024, center A) and external multicenter (81 lobes, center B) test cohorts were incorporated to enhance generalizability. Thyroid lobes were segmented along the isthmus midline, with segmentation reliability confirmed by an intraclass correlation coefficient (≥ 0.80). Radiomics feature extraction was performed using Pearson correlation analysis followed by least absolute shrinkage and selection operator regression with 10-fold cross-validation. Seven machine learning algorithms were systematically evaluated, with model performance quantified through the area under the receiver operating characteristic curve (AUC), Brier score, decision curve analysis, and DeLong test for comparison with radiologists interpretations. Model interpretability was elucidated using SHapley Additive exPlanations (SHAP).
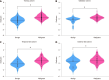
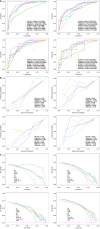
Results: The extreme gradient boosting model demonstrated robust diagnostic performance across all datasets, achieving AUCs of 0.899 [95% confidence interval (CI): 0.845-0.932] in the training cohort, 0.803 (95%CI: 0.715-0.890) in internal validation, 0.855 (95%CI: 0.775-0.935) in temporal testing, and 0.802 (95%CI: 0.664-0.939) in external testing. These results were significantly superior to radiologists assessments (AUCs: 0.596, 0.529, 0.558, and 0.538, respectively; P < 0.001 by DeLong test). SHAP analysis identified radiomic score, age, tumor size stratification, calcification status, and cystic components as key predictive features. The model exhibited excellent calibration (Brier scores: 0.125-0.144) and provided significant clinical net benefit at decision thresholds exceeding 20%, as evidenced by decision curve analysis.
Conclusion: The non-contrast computed tomography-based radiomics-clinical fusion model enables robust preoperative thyroid nodule classification, with SHAP-driven interpretability enhancing its clinical applicability for personalized decision-making.
Keywords: Machine learning; Non-contrast computed tomography; Papillary thyroid carcinoma; Radiomics; Thyroid nodules.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


References
-
- Alexander EK, Cibas ES. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol. 2022;10:533–539. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

